Anti-invasive efficacy and survival benefit of the YAP-TEAD inhibitor verteporfin in preclinical glioblastoma models
- PMID: 34657158
- PMCID: PMC9071332
- DOI: 10.1093/neuonc/noab244
Anti-invasive efficacy and survival benefit of the YAP-TEAD inhibitor verteporfin in preclinical glioblastoma models
Abstract
Background: Glioblastoma (GBM) remains a largely incurable disease as current therapy fails to target the invasive nature of glioma growth in disease progression and recurrence. Here, we use the FDA-approved drug and small molecule Hippo inhibitor Verteporfin (VP) to target YAP-TEAD activity, known to mediate convergent aspects of tumor invasion/metastasis, and assess the drug's efficacy and survival benefit in GBM models.
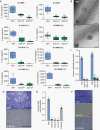
Methods: Up to 8 low-passage patient-derived GBM cell lines with distinct genomic drivers, including 3 primary/recurrent pairs, were treated with VP or vehicle (VEH) to assess in vitro effects on proliferation, migration, invasion, YAP-TEAD activity, and transcriptomics. Patient-derived orthotopic xenograft (PDX) models were used to assess VP's brain penetrance and effects on tumor burden and survival.
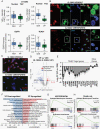
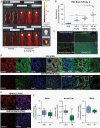
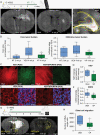
Results: VP treatment disturbed YAP/TAZ-TEAD activity; disrupted transcriptome signatures related to invasion, epithelial-to-mesenchymal, and proneural-to-mesenchymal transition, phenocopying TEAD1-knockout effects; and impaired tumor migration/invasion dynamics across primary and recurrent GBM lines. In an aggressive orthotopic PDX GBM model, short-term VP treatment consistently diminished core and infiltrative tumor burden, which was associated with decreased tumor expression of Ki67, nuclear YAP, TEAD1, and TEAD-associated targets EGFR, CDH2, and ITGB1. Finally, long-term VP treatment appeared nontoxic and conferred survival benefit compared to VEH in 2 PDX models: as monotherapy in primary (de novo) GBM and in combination with Temozolomide chemoradiation in recurrent GBM, where VP treatment associated with increased MGMT methylation.
Conclusions: We demonstrate combined anti-invasive and anti-proliferative efficacy for VP with survival benefit in preclinical GBM models, indicating potential therapeutic value of this already FDA-approved drug if repurposed for GBM patients.
Keywords: Verteporfin; YAP-TEAD; invasion; migration; preclinical.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Comment in
-
Repurposing the drug verteporfin as anti-neoplastic therapy for glioblastoma.Neuro Oncol. 2022 May 4;24(5):708-710. doi: 10.1093/neuonc/noac019. Neuro Oncol. 2022. PMID: 35100422 Free PMC article. No abstract available.
References
-
- Brat DJ, Castellano-Sanchez AA, Hunter SB, et al. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64(3):920–927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

