First-line treatment options for advanced non-small cell lung cancer patients with PD-L1 ≥ 50%: a systematic review and network meta-analysis
- PMID: 34657171
- PMCID: PMC10991470
- DOI: 10.1007/s00262-021-03089-x
First-line treatment options for advanced non-small cell lung cancer patients with PD-L1 ≥ 50%: a systematic review and network meta-analysis
Abstract
Introduction: Single-agent immune checkpoint inhibitors (ICIs) like pembrolizumab or atezolizumab have been approved as first-line monotherapy for advanced non-small cell lung cancer (NSCLC) patients with PD-L1 ≥ 50%. However, emerging evidences have showed that ICI combinations (chemoimmunotherapy or dual-agent ICIs) argue to offer a higher response rate. In this network meta-analysis, we aimed to evaluate the efficacy and toxicity of first-line single-agent ICIs versus ICI combinations for advanced NSCLC patients with PD-L1 ≥ 50%.
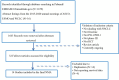
Methods: PubMed, Embase, Cochrane Library and the Clinicaltrials.gov were systematically searched to extract eligible literature until December 2020. Outcomes included overall survival (OS), progression free survival (PFS), objective response rate (ORR) and treatment related adverse events (TRAEs) of grades 3-5.
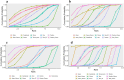
Results: Fourteen studies with 3448 patients were included. The results showed that chemotherapy plus ICIs significantly improved PFS and ORR compared to chemotherapy, and sinti-chemo (HR: 0.31, 95% CI: 0.20-0.49) and pembro-chemo (OR: 4.2, 95% CI: 2.6-6.7) ranked first. In terms of OS, cemiplimab provided the best benefit versus chemotherapy (HR: 0.57, 95% CI: 0.43-0.77), followed by atezolizumab and pembro-chemo. In the subgroup analysis of histological type, pembro-chemo and sinti-chemo showed the best benefit of PFS in squamous and nonsquamous NSCLC, respectively, while there was no significant difference between ICI combinations with single-agent ICIs in OS. Moreover, the addition of chemotherapy to ICIs elevated toxicity compared to chemotherapy.
Conclusion: The study suggested that chemotherapy plus ICIs might improve PFS and ORR than single-agent ICIs for advanced NSCLC patients with PD-L1 ≥ 50%. However, it did not lead to OS benefit.
Keywords: Immune checkpoint inhibitors; Network meta-analysis; Non-small cell lung cancer; PD-(L)1 inhibitors; PD-L1 high expression.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Efficacy and safety of immune checkpoint inhibitors for advanced non-small cell lung cancer with or without PD-L1 selection: A systematic review and network meta-analysis.Chin Med J (Engl). 2023 Sep 20;136(18):2156-2165. doi: 10.1097/CM9.0000000000002750. Epub 2023 Aug 18. Chin Med J (Engl). 2023. PMID: 37596898 Free PMC article.
-
Comparison of efficacy and safety of PD-1/PD-L1 combination therapy in first-line treatment of advanced NSCLC: an updated systematic review and network meta-analysis.Clin Transl Oncol. 2024 Oct;26(10):2488-2502. doi: 10.1007/s12094-024-03442-3. Epub 2024 Apr 16. Clin Transl Oncol. 2024. PMID: 38625495
-
Comparison of Efficacy and Safety of Single and Double Immune Checkpoint Inhibitor-Based First-Line Treatments for Advanced Driver-Gene Wild-Type Non-Small Cell Lung Cancer: A Systematic Review and Network Meta-Analysis.Front Immunol. 2021 Aug 16;12:731546. doi: 10.3389/fimmu.2021.731546. eCollection 2021. Front Immunol. 2021. PMID: 34484242 Free PMC article.
-
Efficacy and safety of first-line PD-1/PD-L1 inhibitors combined with or without anti-angiogenesis therapy for extensive-stage small-cell lung cancer: a network meta-analysis.Ther Adv Med Oncol. 2025 Jun 25;17:17588359251348310. doi: 10.1177/17588359251348310. eCollection 2025. Ther Adv Med Oncol. 2025. PMID: 40574965 Free PMC article.
-
Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis.Lung Cancer. 2019 Aug;134:127-140. doi: 10.1016/j.lungcan.2019.05.029. Epub 2019 May 30. Lung Cancer. 2019. PMID: 31319971
Cited by
-
The efficacy and safety of immune-checkpoint inhibitors plus chemotherapy versus chemotherapy for non-small cell lung cancer: An updated systematic review and meta-analysis.PLoS One. 2024 Feb 6;19(2):e0276318. doi: 10.1371/journal.pone.0276318. eCollection 2024. PLoS One. 2024. PMID: 38319920 Free PMC article.
-
Unraveling the influence of TTF-1 expression on immunotherapy outcomes in PD-L1-high non-squamous NSCLC: a retrospective multicenter study.Front Immunol. 2024 Jul 15;15:1399889. doi: 10.3389/fimmu.2024.1399889. eCollection 2024. Front Immunol. 2024. PMID: 39076994 Free PMC article.
-
Combining PD-1 or PD-L1 inhibitors with chemotherapy is a good strategy for the treatment of extensive small cell lung cancer: A retrospective analysis of clinical studies.Front Immunol. 2022 Dec 5;13:1059557. doi: 10.3389/fimmu.2022.1059557. eCollection 2022. Front Immunol. 2022. PMID: 36544769 Free PMC article.
-
First-line treatment options for advanced gastric/gastroesophageal junction cancer patients with PD-L1-positive: a systematic review and meta-analysis.Ann Med Surg (Lond). 2023 May 3;85(6):2875-2883. doi: 10.1097/MS9.0000000000000765. eCollection 2023 Jun. Ann Med Surg (Lond). 2023. PMID: 37363517 Free PMC article.
-
Genome-Wide Methylation Analysis in Two Wild-Type Non-Small Cell Lung Cancer Subgroups with Negative and High PD-L1 Expression.Cancers (Basel). 2024 May 11;16(10):1841. doi: 10.3390/cancers16101841. Cancers (Basel). 2024. PMID: 38791918 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

