Memantine Improves Recovery After Spreading Depolarization in Brain Slices and can be Considered for Future Clinical Trials
- PMID: 34657268
- PMCID: PMC9938764
- DOI: 10.1007/s12028-021-01351-9
Memantine Improves Recovery After Spreading Depolarization in Brain Slices and can be Considered for Future Clinical Trials
Abstract
Background: Spreading depolarization (SD) has been identified as a key mediator of secondary lesion progression after acute brain injuries, and clinical studies are beginning to pharmacologically target SDs. Although initial work has focused on the N-Methyl-D-aspartate receptor antagonist ketamine, there is also interest in alternatives that may be better tolerated. We recently showed that ketamine can inhibit mechanisms linked to deleterious consequences of SD in brain slices. The present study tested the hypothesis that memantine improves recovery of brain slices after SD and explored the effects of memantine in a clinical case targeting SD.
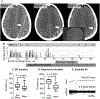
Methods: For mechanistic studies, electrophysiological and optical recordings were made from hippocampal area CA1 in acutely prepared brain slices from mice. SDs were initiated by localized microinjection of K+ in conditions of either normal or reduced metabolic substrate availability. Memantine effects were assessed from intrinsic optical signals and extracellular potential recordings. For the clinical report, a subdural strip electrode was used for continuous electrocorticographic recording after the surgical evacuation of a chronic subdural hematoma.
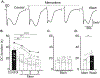
Results: In brain slice studies, memantine (10-300 µM) did not prevent the initiation of SD, but impaired SD propagation rate and recovery from SD. Memantine reduced direct current (DC) shift duration and improved recovery of synaptic potentials after SD. In brain slices with reduced metabolic substrate availability, memantine reduced the evidence of structural disruption after the passage of SD. In our clinical case, memantine did not noticeably immediately suppress SD; however, it was associated with a significant reduction of SD duration and a reduction in the electrocorticographic (ECoG) suppression that occurs after SD. SD was completely suppressed, with improvement in neurological examination with the addition of a brief course of ketamine.
Conclusions: These data extend recent work showing that N-Methyl-D-aspartate receptor antagonists can improve recovery from SD. These results suggest that memantine could be considered for future clinical trials targeting SD, and in some cases as an adjunct or alternative to ketamine.
Keywords: Brain injury; Cortical spreading depression; Excitotoxin; Kemantine; Ketamine; NMDA receptors; Subdural hematoma.
© 2021. Springer Science+Business Media, LLC, part of Springer Nature and Neurocritical Care Society.
Figures



Similar articles
-
Ketamine improves neuronal recovery following spreading depolarization in peri-infarct tissues.J Neurochem. 2024 May;168(5):855-867. doi: 10.1111/jnc.15923. Epub 2023 Aug 18. J Neurochem. 2024. PMID: 37596720 Free PMC article.
-
The NMDA receptor antagonists memantine and ketamine as anti-migraine agents.Naunyn Schmiedebergs Arch Pharmacol. 2023 Jul;396(7):1371-1398. doi: 10.1007/s00210-023-02444-2. Epub 2023 Mar 4. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 36869904 Review.
-
Ketamine reduces deleterious consequences of spreading depolarizations.Exp Neurol. 2018 Jul;305:121-128. doi: 10.1016/j.expneurol.2018.04.007. Epub 2018 Apr 10. Exp Neurol. 2018. PMID: 29653188 Free PMC article.
-
Memantine and Ketamine Differentially Alter NMDA Receptor Desensitization.J Neurosci. 2017 Oct 4;37(40):9686-9704. doi: 10.1523/JNEUROSCI.1173-17.2017. Epub 2017 Sep 6. J Neurosci. 2017. PMID: 28877967 Free PMC article.
-
Mechanism of action of memantine.Curr Opin Pharmacol. 2006 Feb;6(1):61-7. doi: 10.1016/j.coph.2005.09.007. Epub 2005 Dec 20. Curr Opin Pharmacol. 2006. PMID: 16368266 Review.
Cited by
-
Brain Tsunamis in Human High-Grade Glioma: Preliminary Observations.Brain Sci. 2022 May 30;12(6):710. doi: 10.3390/brainsci12060710. Brain Sci. 2022. PMID: 35741596 Free PMC article.
-
Altered Cortical Trigeminal Fields Excitability by Spreading Depolarization Revealed with in Vivo Functional Ultrasound Imaging Combined with Electrophysiology.J Neurosci. 2022 Aug 10;42(32):6295-6308. doi: 10.1523/JNEUROSCI.1825-21.2022. J Neurosci. 2022. PMID: 35817577 Free PMC article.
-
Ketamine improves neuronal recovery following spreading depolarization in peri-infarct tissues.J Neurochem. 2024 May;168(5):855-867. doi: 10.1111/jnc.15923. Epub 2023 Aug 18. J Neurochem. 2024. PMID: 37596720 Free PMC article.
-
Pathophysiology of Early Brain Injury and Its Association with Delayed Cerebral Ischemia in Aneurysmal Subarachnoid Hemorrhage: A Review of Current Literature.J Clin Med. 2023 Jan 28;12(3):1015. doi: 10.3390/jcm12031015. J Clin Med. 2023. PMID: 36769660 Free PMC article. Review.
-
The NMDA receptor antagonists memantine and ketamine as anti-migraine agents.Naunyn Schmiedebergs Arch Pharmacol. 2023 Jul;396(7):1371-1398. doi: 10.1007/s00210-023-02444-2. Epub 2023 Mar 4. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 36869904 Review.
References
-
- Leao AAP. Spreading depression of activity in the cerebral cortex. J Neurophysiol 1944;7:359–90. - PubMed
-
- Leao AAP. Further observations on the spreading depression of activity in the cerebral cortex. J Neurophysiol 1947;10:409–14. - PubMed
-
- Hartings JA, Shuttleworth CW, Kirov SA, et al. The continuum of spreading depolarizations in acute cortical lesion development: Examining Leao’s legacy. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2017;37:1571–94. - PMC - PubMed
-
- Hinzman JM, DiNapoli VA, Mahoney EJ, Gerhardt GA, Hartings JA. Spreading depolarizations mediate excitotoxicity in the development of acute cortical lesions. Experimental neurology 2015;267:243–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

