Efficacy, Safety and Immunogenicity of AVT02 Versus Originator Adalimumab in Subjects with Moderate to Severe Chronic Plaque Psoriasis: A Multicentre, Double-Blind, Randomised, Parallel Group, Active Control, Phase III Study
- PMID: 34657274
- PMCID: PMC8520467
- DOI: 10.1007/s40259-021-00502-w
Efficacy, Safety and Immunogenicity of AVT02 Versus Originator Adalimumab in Subjects with Moderate to Severe Chronic Plaque Psoriasis: A Multicentre, Double-Blind, Randomised, Parallel Group, Active Control, Phase III Study
Abstract
Background: AVT02 (adalimumab) is a proposed biosimilar to Humira®. AVT02 is produced at a 100 mg/mL concentration with a citrate-free formulation.
Objectives: The aim of this study was to compare the efficacy, safety and immunogenicity of AVT02 versus Humira® in subjects with moderate to severe chronic plaque psoriasis.
Methods: This double-blind, randomised, parallel group, active control study of adult subjects compared (at a 1:1 ratio) AVT02 with originator adalimumab 80 mg subcutaneously in Week 1, then 40 mg every other week. At Week 16, subjects who had received originator adalimumab were re-randomised at a 1:1 ratio to continue receiving originator adalimumab, or to switch to AVT02, every other week until Week 48, with final efficacy endpoint at Week 50. Subjects who initially received AVT02 continued to receive AVT02 from Week 16 to Week 48. The primary endpoint was percentage improvement in Psoriasis Area and Severity Index (PASI) score at Week 16. Secondary efficacy endpoints included percentage improvement in PASI score at additional timepoints, change from baseline in Dermatology Life Quality Index (DLQI) score and number and percentage of subjects achieving static Physician's Global Assessment (sPGA) responses of 'clear' or 'almost clear'. Additional secondary endpoints included comparison of adverse event profiles, anti-drug antibodies and neutralising antibodies, and serum trough levels of adalimumab at steady state.
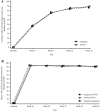
Results: A total of 413 subjects were randomised (205 to AVT02 and 208 to originator). The percentage improvement in PASI score at Week 16 was 91.6% for AVT02-treated subjects and 89.6% for originator adalimumab. The 90% confidence intervals for the primary endpoint were within the pre-defined equivalence margin of ±10% (90% CI - 0.76 to 5.29; 95% CI - 1.34 to 5.88), and a comparable pattern for DLQI score (11.4-point and 10.6-point improvement in AVT02-treated and originator adalimumab-treated groups, respectively) and sPGA (90.5% in both groups achieving 'clear' or 'almost clear') at Week 16 supported the assessment. Efficacy persisted through Week 50 of the study in all treatment groups, including those who switched from originator adalimumab to AVT02, for percent improvement in PASI score, quality-of-life assessment and sPGA. The safety, tolerability and immunogenicity profiles between AVT02 and originator adalimumab were similar at Week 16, and this persisted in the switched and continued groups through Week 50.
Conclusion: Objective and subjective measures of efficacy supported the evaluation of biosimilarity between AVT02 and originator adalimumab at Week 16 and until Week 50, in switched and continued treatment groups. AVT02 was safe and well tolerated, with a safety and immunogenicity profile similar to that observed in originator adalimumab with no clinically meaningful difference between the two.
Clinical trial registration: EudraCT: 2017-003367-35; ClinicalTrials.gov: NCT03849404.
© 2021. The Author(s).
Conflict of interest statement
FB, JS, RD, EG, HO, HNH and HS are employees at Alvotech. SF has received research grants from Abbvie, Janssen, Lilly and Novartis and speaker honoraria from Alvotech, Abbvie, Amgen, Lilly, Novartis and Janssen. RK's company has received consultancy fees in relation to this study and in other studies conducted by Alvotech, but no consultancy fees have been received in relation to the writing of this manuscript. NR, GP, KK, and GG declare that they have no conflicts of interest that might be relevant to this work.
Figures


References
-
- EMA. Humira (adalimumab). Summary of Product Characteristics 2021. https://www.ema.europa.eu/en/documents/product-information/humira-epar-p.... Accessed 12 Aug 2021.
-
- FDA. Humira (adalimumab) US Package Insert. AbbVie, Inc., 2021. https://www.rxabbvie.com/pdf/humira.pdf. Accessed 12 Aug 2021.
-
- Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008; 117(2): 244-279. Doi: 10.1016/j.pharmthera.2007.10.001. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

