Efficacy and Safety of Sarilumab in patients with COVID19 Pneumonia: A Randomized, Phase III Clinical Trial (SARTRE Study)
- PMID: 34658006
- PMCID: PMC8520759
- DOI: 10.1007/s40121-021-00543-2
Efficacy and Safety of Sarilumab in patients with COVID19 Pneumonia: A Randomized, Phase III Clinical Trial (SARTRE Study)
Abstract
Introduction: SARS-CoV-2 pneumonia is often associated with hyper-inflammation. The cytokine-storm-like is one of the targets of current therapies for coronavirus disease 2019 (COVID-19). High Interleukin-6 (IL6) blood levels have been identified in severe COVID-19 disease, but there are still uncertainties regarding the actual role of anti-IL6 antagonists in COVID-19 management. Our hypothesis was that the use of sarilumab plus corticosteroids at an early stage of the hyper-inflammatory syndrome would be beneficial and prevent progression to acute respiratory distress syndrome (ARDS).
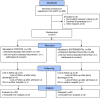
Methods: We randomly assigned (in a 1:1 ratio) COVID-19 pneumonia hospitalized patients under standard oxygen therapy and laboratory evidence of hyper-inflammation to receive sarilumab plus usual care (experimental group) or usual care alone (control group). Corticosteroids were given to all patients at a 1 mg/kg/day of methylprednisolone for at least 3 days. The primary outcome was the proportion of patients progressing to severe respiratory failure (defined as a score in the Brescia-COVID19 scale ≥ 3) up to day 15.
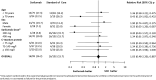
Results: A total of 201 patients underwent randomization: 99 patients in the sarilumab group and 102 patients in the control group. The rate of patients progressing to severe respiratory failure (Brescia-COVID scale score ≥ 3) up to day 15 was 16.16% in the Sarilumab group versus 15.69% in the control group (RR 1.03; 95% CI 0.48-2.20). No relevant safety issues were identified.
Conclusions: In hospitalized patients with Covid-19 pneumonia, who were under standard oxygen therapy and who presented analytical inflammatory parameters, an early therapeutic intervention with sarilumab plus standard of care (including corticosteroids) was not shown to be more effective than current standard of care alone. The study was registered at EudraCT with number: 2020-002037-15.
Keywords: COVID19; Corticosteroids; IL6-inhibitors; Randomized clinical trial; Sarilumab.
© 2021. The Author(s).
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous