Mechanism of the enhancing effect of glycyrrhizin on nifedipine penetration through a lipid membrane
- PMID: 34658466
- PMCID: PMC8500845
- DOI: 10.1016/j.molliq.2021.117759
Mechanism of the enhancing effect of glycyrrhizin on nifedipine penetration through a lipid membrane
Abstract
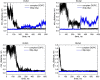
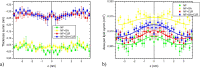
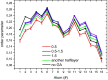
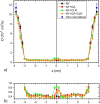
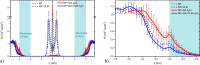
The saponin glycyrrhizin from liquorice root shows the ability to enhance the therapeutic activity of other drugs when used as a drug delivery system. Due to its amphiphilic properties, glycyrrhizin can form self-associates (dimers, micelles) and supramolecular complexes with a wide range of hydrophobic drugs, which leads to an increase in their solubility, stability and bioavailability. That is why the mechanism of the biological activity of glycyrrhizin is of considerable interest and has been the subject of intensive physical and chemical research in the last decade. Two mechanisms have been proposed to explain the effect of glycyrrhizin on drug bioavailability, namely, the increase in drug solubility in water and enhancement of the membrane permeability. Interest in the membrane-modifying ability of glycyrrhizic acid (GA) is also growing at present due to its recently discovered antiviral activity against SARS-CoV-2 Bailly and Vergoten (2020) [1]. In the present study, the passive permeability of the DOPC lipid membrane for the calcium channel blocker nifedipine was elucidated by parallel artificial membrane permeability assay (PAMPA) and full atomistic molecular dynamics (MD) simulation with free energy calculations. PAMPA experiments show a remarkable increase in the amount of nifedipine (NF) permeated with glycyrrhizin compared to free NF. In previous studies, we have shown using MD techniques that glycyrrhizin molecules can integrate into the lipid bilayer. In this study, MD simulation demonstrates a significant decrease in the energy barrier of NF penetration through the lipid bilayer in the presence of glycyrrhizin both in the pure DOPC membrane and in the membrane with cholesterol. This effect can be explained by the formation of hydrogen bonds between NF and GA in the middle of the bilayer.
Keywords: CLR, cholesterol; DDS, drug delivery system; DOPC; DOPC, dioleoylphosphatidylcholine; Drug delivery; GA, glycyrrhizic acid; Glycyrrhizin; Lipid bilayer; MD, molecular dynamics; Membrane penetration; Molecular dynamics; NF, nifedipine; NMR; NMR, nuclear magnetic resonance; Nifedipine; PAMPA; PAMPA, parallel artificial membrane permeability assay; PMF, potential of mean force; TBK, tebuconazole; VDW, Van der Waals.
© 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




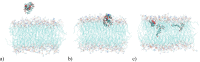






References
-
- Tekade R.K., Maheshwari R., Tekade M. Biopolymer-Based Composites. Elsevier; 2017. pp. 81–106. - DOI
-
- Dushkin A.V., Tolstikova T.G., Khvostov M.V., Tolstikov G.A. In: The Complex World of Polysaccharides. Karunaratne D.N., editor. InTech, RijdrugCroatia; 2012. Complexes of polysaccharides and glycyrrhizic acid with drug molecules. Mechanochemical synthesis and pharmacological activity; pp. 573–602. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
