GluN2A/ERK/CREB Signaling Pathway Involved in Electroacupuncture Regulating Hypothalamic-Pituitary-Adrenal Axis Hyperactivity
- PMID: 34658758
- PMCID: PMC8514998
- DOI: 10.3389/fnins.2021.703044
GluN2A/ERK/CREB Signaling Pathway Involved in Electroacupuncture Regulating Hypothalamic-Pituitary-Adrenal Axis Hyperactivity
Abstract
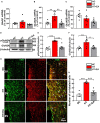
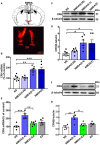
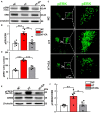
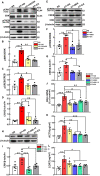
The hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis caused by stress will inevitably disrupt the homeostasis of the neuroendocrine system and damage physiological functions. It has been demonstrated that electroacupuncture (EA) can modulate HPA axis hyperactivity during the perioperative period. As the initiating factor of the HPA axis, hypothalamic corticotrophin-releasing hormone (CRH) is the critical molecule affected by EA. However, the mechanism by which EA reduces CRH synthesis and secretion remains unclear. Activated N-methyl-D-aspartate receptor (NMDAR) has been linked to over-secretion of hypothalamic CRH induced by stress. To determine whether NMDAR is involved in EA regulating the over-expression of CRH, a surgical model of partial hepatectomy (HT) was established in our experiment. The effect of EA on hypothalamic NMDAR expression in HT mice was examined. Then, we investigated whether the extracellular regulated protein kinases (ERK)/cyclic adenosine monophosphate response element-binding protein (CREB) signaling pathway mediated by NMDAR was involved in EA regulating HPA axis hyperactivity. It was found that surgery enhanced the expression of hypothalamic CRH and caused HPA axis hyperactivity. Intriguingly, EA effectively suppressed the expression of CRH and decreased the activation of GluN2A (NMDAR subunit), ERK, and CREB in HT mice. GluN2A, ERK, and CREB antagonists had similar effects on normalizing the expression of CRH and HPA axis function compared with EA. Our findings suggested that surgery enhanced the activation of the hypothalamic GluN2A/ERK/CREB signaling pathway, thus promoting the synthesis and secretion of CRH. EA suppressed the phosphorylation of GluN2A, ERK, and CREB in mice that had undergone surgery, indicating that the GluN2A/ERK/CREB signaling pathway was involved in EA alleviating HPA axis hyperactivity.
Keywords: CRH; GluN2A/ERK/CREB signaling pathway; HPA axis; electroacupuncture; surgical trauma.
Copyright © 2021 Wang, Han, Zhu, Zhang, Ju, Du and Tian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Electroacupuncture Ameliorates Hypothalamic‒Pituitary‒Adrenal Axis Dysfunction Induced by Surgical Trauma in Mice Through the Hypothalamic Oxytocin System.Neurochem Res. 2023 Nov;48(11):3391-3401. doi: 10.1007/s11064-023-03984-y. Epub 2023 Jul 12. Neurochem Res. 2023. PMID: 37436613
-
Electroacupuncture negatively regulates the Nesfatin-1/ERK/CREB pathway to alleviate HPA axis hyperactivity and anxiety-like behaviors caused by surgical trauma.Chin Med. 2024 Aug 17;19(1):108. doi: 10.1186/s13020-024-00974-2. Chin Med. 2024. PMID: 39153974 Free PMC article.
-
Electroacupuncture Alleviates Surgical Trauma-Induced Hypothalamus Pituitary Adrenal Axis Hyperactivity Via microRNA-142.Front Mol Neurosci. 2017 Sep 27;10:308. doi: 10.3389/fnmol.2017.00308. eCollection 2017. Front Mol Neurosci. 2017. PMID: 29021740 Free PMC article.
-
Neuroendocrine perturbations in fibromyalgia and chronic fatigue syndrome.Rheum Dis Clin North Am. 2000 Nov;26(4):989-1002. doi: 10.1016/s0889-857x(05)70180-0. Rheum Dis Clin North Am. 2000. PMID: 11084955 Review.
-
The stress system in the human brain in depression and neurodegeneration.Ageing Res Rev. 2005 May;4(2):141-94. doi: 10.1016/j.arr.2005.03.003. Ageing Res Rev. 2005. PMID: 15996533 Review.
Cited by
-
Electroacupuncture alleviates perioperative hypothalamus-pituitary-adrenal axis dysfunction via circRNA-miRNA-mRNA networks.Front Mol Neurosci. 2023 Jan 25;16:1115569. doi: 10.3389/fnmol.2023.1115569. eCollection 2023. Front Mol Neurosci. 2023. PMID: 36760604 Free PMC article.
-
Electroacupuncture Ameliorates Hypothalamic‒Pituitary‒Adrenal Axis Dysfunction Induced by Surgical Trauma in Mice Through the Hypothalamic Oxytocin System.Neurochem Res. 2023 Nov;48(11):3391-3401. doi: 10.1007/s11064-023-03984-y. Epub 2023 Jul 12. Neurochem Res. 2023. PMID: 37436613
-
Electroacupuncture negatively regulates the Nesfatin-1/ERK/CREB pathway to alleviate HPA axis hyperactivity and anxiety-like behaviors caused by surgical trauma.Chin Med. 2024 Aug 17;19(1):108. doi: 10.1186/s13020-024-00974-2. Chin Med. 2024. PMID: 39153974 Free PMC article.
References
-
- Cai Y. Y., Liu Z. S., Wang S., Wang Q. F., Cui X. M., Qu L. (2009). [Influence of electroacupuncture of meridian acupoints on the related hormones of the hypothalamus-pituitary-adrenal axis in rats with cerebral ischemia reperfusion injury]. Zhen. Ci. Yan. Jiu. 34 297–303. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

