Peripheral Clock System Abnormalities in Patients With Parkinson's Disease
- PMID: 34658839
- PMCID: PMC8519399
- DOI: 10.3389/fnagi.2021.736026
Peripheral Clock System Abnormalities in Patients With Parkinson's Disease
Abstract
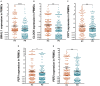
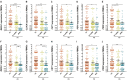
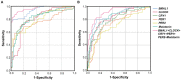
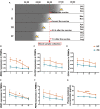
Objective: To evaluate the altered expression of peripheral clock genes, circulating melatonin levels, and their correlations with sleep-wake phenotypes including probable rapid eye movement sleep behavior disorder (pRBD) symptoms in a relatively large population of Parkinson's disease (PD) patients. Methods: We determined the expression profiles of five principal clock genes, BMAL1, CLOCK, CRY1, PER1, and PER2, in the peripheral blood mononuclear cells (PBMCs) of PD patients (n = 326), and healthy controls (HC, n = 314) using quantitative real-time PCR. Melatonin concentration in the plasma of two groups was evaluated by enzyme-linked immunosorbent assay. Then we performed comprehensive association analyses on the PBMCs clock gene expression, plasma melatonin levels and sleep characteristics. Results: Our data showed that the expression levels of BMAL1, CLOCK, CRY1, PER1, and PER2 were significantly decreased in the PBMCs of PD as compared with that of HC (P < 0.05). PD patients had reduced plasma melatonin levels compared with HC (P < 0.0001). pRBD and excessive daytime sleepiness are common in these PD patients and are associated with the expression levels of all five clock genes (r = -0.344∼-0.789, P < 0.01) and melatonin concentration (r = -0.509∼-0.753, P < 0.01). Statistical analyses also revealed that a combination of five clock genes and melatonin could reach a high diagnostic performance (areas under the curves, 97%) for PD comorbid pRBD. Conclusion: This case-control study demonstrates that peripheral BMAL1, CLOCK, CRY1, PER1, PER2, and melatonin levels are altered in PD patients and may serve as endogenous markers for sleep and wakefulness disturbances of PD.
Keywords: Parkinson’s disease; circadian rhythm; clock gene; melatonin; sleep-wake disturbances.
Copyright © 2021 Li, Cheng, Jia, Leng, Qian, Yu, Liu, Wang, Yang, Al-Nusaif and Le.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Effect of melatonin administration on the PER1 and BMAL1 clock genes in patients with Parkinson's disease.Biomed Pharmacother. 2020 Sep;129:110485. doi: 10.1016/j.biopha.2020.110485. Epub 2020 Jul 6. Biomed Pharmacother. 2020. PMID: 32768967 Clinical Trial.
-
The Instigation of the Associations Between Melatonin, Circadian Genes, and Epileptic Spasms in Infant Rats.Front Neurol. 2020 Oct 26;11:497225. doi: 10.3389/fneur.2020.497225. eCollection 2020. Front Neurol. 2020. PMID: 33192961 Free PMC article.
-
Altered circadian clock gene expression in the sperm of infertile men with asthenozoospermia.J Assist Reprod Genet. 2022 Jan;39(1):165-172. doi: 10.1007/s10815-021-02375-y. Epub 2022 Jan 9. J Assist Reprod Genet. 2022. PMID: 35000095 Free PMC article.
-
Neuroprotective Effect of Melatonin on Sleep Disorders Associated with Parkinson's Disease.Antioxidants (Basel). 2023 Feb 6;12(2):396. doi: 10.3390/antiox12020396. Antioxidants (Basel). 2023. PMID: 36829955 Free PMC article. Review.
-
Melatonin feedback on clock genes: a theory involving the proteasome.J Pineal Res. 2015 Jan;58(1):1-11. doi: 10.1111/jpi.12189. Epub 2014 Nov 22. J Pineal Res. 2015. PMID: 25369242 Review.
Cited by
-
Transcriptome Profiling Reveals Differential Expression of Circadian Behavior Genes in Peripheral Blood of Monozygotic Twins Discordant for Parkinson's Disease.Cells. 2022 Aug 20;11(16):2599. doi: 10.3390/cells11162599. Cells. 2022. PMID: 36010675 Free PMC article.
-
Sleep Disturbance in Parkinson's Disease: Consequences for the Brain and Disease Progression - A Narrative Review.Nat Sci Sleep. 2025 Jun 28;17:1521-1537. doi: 10.2147/NSS.S478860. eCollection 2025. Nat Sci Sleep. 2025. PMID: 40606508 Free PMC article. Review.
-
Circadian re-set repairs long-COVID in a prodromal Parkinson's parallel: a case series.J Med Case Rep. 2024 Oct 23;18(1):496. doi: 10.1186/s13256-024-04812-9. J Med Case Rep. 2024. PMID: 39438926 Free PMC article.
-
Melatonin as an Anti-Aging Therapy for Age-Related Cardiovascular and Neurodegenerative Diseases.Front Aging Neurosci. 2022 Jun 3;14:888292. doi: 10.3389/fnagi.2022.888292. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35721030 Free PMC article. Review.
-
Neuroprotective Potential of Melatonin: Evaluating Therapeutic Efficacy in Alzheimer's and Parkinson's Diseases.Cureus. 2023 Dec 22;15(12):e50948. doi: 10.7759/cureus.50948. eCollection 2023 Dec. Cureus. 2023. PMID: 38259379 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

