Therapeutic Effect of Combining Anisodamine With Neostigmine on Local Scar Formation Following Roux-en-Y Choledochojejunostomy in a Novel Rat Model
- PMID: 34658849
- PMCID: PMC8511430
- DOI: 10.3389/fphar.2021.700050
Therapeutic Effect of Combining Anisodamine With Neostigmine on Local Scar Formation Following Roux-en-Y Choledochojejunostomy in a Novel Rat Model
Abstract
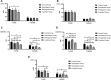
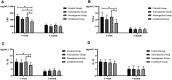
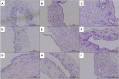
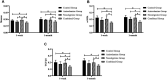
Background: The present study aimed to explore the potential effect of combining anisodamine with neostigmine on local scar formation following Roux-en-Y choledochojejunostomy (RCJS) in a novel rat model. Methods: The biliary obstruction model of Sprague Dawley (SD) rats was established in advance, and 54 rats were divided into nine groups randomly (sham operation group, anisodamine group, neostigmine group, combination group, and control group). Anisodamine (25 mg/kg) and neostigmine (50 μg/kg) were injected to the abdominal cavity separately or simultaneously for 1 week since the first day after surgery according to their allocated intervention, while the same amount of saline (0.5 ml) was injected intraperitoneally in the control group. Indexes including body weight, the diameter of the common bile duct, liver function, inflammatory indexes, and the condition of scar formation in different groups at certain time were evaluated in our study. Results: Recovery of liver function (ALT, AST, TB, DB, and GGT) and systematic inflammation indexes (CRP, TNF-α, and IL-1β) in the combination group was prior to that in the control group (p < 0.05), while no statistical difference in the serum level of IL-10 was observed among groups. Rats in the combination group represented a wider anastomotic diameter and lower expression of α-SMA and TGF-β1 at anastomotic stoma compared to the control group (p < 0.05). Histopathological staining showed slighter proliferation of collagen and smooth muscle fibers in rats' bile duct wall and less local scar formation at anastomotic stoma compared to the control group. Conclusion: The combination of anisodamine and neostigmine can alleviate local and systemic inflammatory response, promote the recovery of liver function, and reduce scar formation in rats after the RCJS procedure.
Keywords: Roux-en-Y choledochojejunostomy; anisodamine; inflammatory response; neostigmine; scar formation.
Copyright © 2021 Lyu, Wang, Xu, Wang, Pan, Jiang, He and Lang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
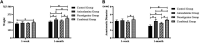



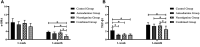
Similar articles
-
Mechanism of scar formation following Roux-en-Y choledochojejunostomy in a novel rat model of obstructive jaundice.Ann Transl Med. 2021 Mar;9(6):456. doi: 10.21037/atm-20-5135. Ann Transl Med. 2021. PMID: 33850853 Free PMC article.
-
IL-10 dependent modulatory effect of regulatory B10 cells on local scar formation following Roux-en-Y choledochojejunostomy in a novel rat model.Int Immunopharmacol. 2024 Jan 5;126:111309. doi: 10.1016/j.intimp.2023.111309. Epub 2023 Dec 4. Int Immunopharmacol. 2024. PMID: 38048666
-
[Clinical effect of nano-fat mixed granule fat transplantation in the treatment of cicatricial facial depression and atrophy and the related experimental mechanism].Zhonghua Shao Shang Za Zhi. 2019 Apr 20;35(4):266-276. doi: 10.3760/cma.j.issn.1009-2587.2019.04.006. Zhonghua Shao Shang Za Zhi. 2019. PMID: 31060174 Chinese.
-
Ascending cholangitis provokes IL-8 and MCP-1 expression and promotes inflammatory cell infiltration in the cholestatic rat liver.J Pediatr Surg. 2001 Nov;36(11):1623-8. doi: 10.1053/jpsu.2001.27933. J Pediatr Surg. 2001. PMID: 11685687
-
Treatment of chronic pancreatitis complicated by obstruction of the common bile duct or duodenum.World J Surg. 1990 Jan-Feb;14(1):59-69. doi: 10.1007/BF01670547. World J Surg. 1990. PMID: 2407039 Review.
Cited by
-
A novel ameliorated rat model of reversible obstructive jaundice.J Zhejiang Univ Sci B. 2023 Mar 25;24(4):345-351. doi: 10.1631/jzus.B2200421. J Zhejiang Univ Sci B. 2023. PMID: 37056210 Free PMC article.
-
Novel partial common bile duct ligation procedure in rats with reduced mortality and delayed onset of cirrhosis.Exp Ther Med. 2025 Jul 3;30(3):167. doi: 10.3892/etm.2025.12917. eCollection 2025 Sep. Exp Ther Med. 2025. PMID: 40692768 Free PMC article.
-
New advances in clinical application of neostigmine: no longer focusing solely on increasing skeletal muscle strength.Front Pharmacol. 2023 Aug 4;14:1227496. doi: 10.3389/fphar.2023.1227496. eCollection 2023. Front Pharmacol. 2023. PMID: 37601044 Free PMC article. Review.
References
-
- Booij K. A. C., Coelen R. J., de Reuver P. R., Besselink M. G., van Delden O. M., Rauws E. A., et al. (2018). Long-term Follow-Up and Risk Factors for Strictures after Hepaticojejunostomy for Bile Duct Injury: An Analysis of Surgical and Percutaneous Treatment in a Tertiary center. Surgery 163 (5), 1121–1127. 10.1016/j.surg.2018.01.003 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

