The Manifold Roles of Sphingolipids in Viral Infections
- PMID: 34658908
- PMCID: PMC8511394
- DOI: 10.3389/fphys.2021.715527
The Manifold Roles of Sphingolipids in Viral Infections
Abstract
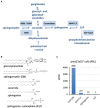
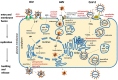
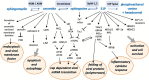
Sphingolipids are essential components of eukaryotic cells. In this review, we want to exemplarily illustrate what is known about the interactions of sphingolipids with various viruses at different steps of their replication cycles. This includes structural interactions during entry at the plasma membrane or endosomal membranes, early interactions leading to sphingolipid-mediated signal transduction, interactions with internal membranes and lipids during replication, and interactions during virus assembly and budding. Targeted interventions in sphingolipid metabolism - as far as they can be tolerated by cells and organisms - may open novel possibilities to support antiviral therapies. Human immunodeficiency virus type 1 (HIV-1) infections have intensively been studied, but for other viral infections, such as influenza A virus (IAV), measles virus (MV), hepatitis C virus (HCV), dengue virus, Ebola virus, and severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), investigations are still in their beginnings. As many inhibitors of sphingolipid metabolism are already in clinical use against other diseases, repurposing studies for applications in some viral infections appear to be a promising approach.
Keywords: ceramide; plasma membrane; sphingolipid; sphingosine-1-phosphate; virus budding; virus entry; virus replication.
Copyright © 2021 Avota, Bodem, Chithelen, Mandasari, Beyersdorf and Schneider-Schaulies.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

