The Role of L-Selectin in HIV Infection
- PMID: 34659153
- PMCID: PMC8511817
- DOI: 10.3389/fmicb.2021.725741
The Role of L-Selectin in HIV Infection
Abstract
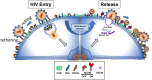
HIV envelope glycoprotein is the most heavily glycosylated viral protein complex identified with over 20 glycans on its surface. This glycan canopy is thought to primarily shield the virus from host immune recognition as glycans are poor immunogens in general, however rare HIV neutralizing antibodies nevertheless potently recognize the glycan epitopes. While CD4 and chemokine receptors have been known as viral entry receptor and coreceptor, for many years the role of viral glycans in HIV entry was controversial. Recently, we showed that HIV envelope glycan binds to L-selectin in solution and on CD4 T lymphocytes. The viral glycan and L-selectin interaction functions to facilitate the viral adhesion and entry. Upon entry, infected CD4 T lymphocytes are stimulated to progressively shed L-selectin and suppressing this lectin receptor shedding greatly reduced HIV viral release and caused aggregation of diminutive virus-like particles within experimental infections and from infected primary T lymphocytes derived from both viremic and aviremic individuals. As shedding of L-selectin is mediated by ADAM metalloproteinases downstream of host-cell stimulation, these findings showed a novel mechanism for HIV viral release and offer a potential new class of anti-HIV compounds.
Keywords: ADAM metalloproteinases; HIV-1 infection; L-selectin (CD62L); envelope gp120; shedding; viral entry; viral release.
Copyright © 2021 Segura, He, Ireland, Zou, Shen, Roth and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

