Coronavirus Nsp1: Immune Response Suppression and Protein Expression Inhibition
- PMID: 34659188
- PMCID: PMC8512706
- DOI: 10.3389/fmicb.2021.752214
Coronavirus Nsp1: Immune Response Suppression and Protein Expression Inhibition
Abstract
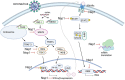
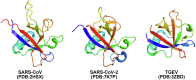
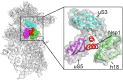
Coronaviruses have brought severe challenges to public health all over the world in the past 20years. SARS-CoV-2, the causative agent of the COVID-19 pandemic that has led to millions of deaths, belongs to the genus beta-coronavirus. Alpha- and beta-coronaviruses encode a unique protein, nonstructural protein 1 (Nsp1) that both suppresses host immune responses and reduces global gene expression levels in the host cells. As a key pathogenicity factor of coronaviruses, Nsp1 redirects the host translation machinery to increase synthesis of viral proteins. Through multiple mechanisms, coronaviruses impede host protein expression through Nsp1, while escaping inhibition to allow the translation of viral RNA. In this review, we discuss current data about suppression of the immune responses and inhibition of protein synthesis induced by coronavirus Nsp1, as well as the prospect of live-attenuated vaccine development with virulence-attenuated viruses with mutations in Nsp1.
Keywords: coronavirus; immune suppression; nonstructural protein 1; translation inhibition; vaccine.
Copyright © 2021 Yuan, Balaji, Lomakin and Xiong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Almeida M. S., Johnson M. A., Herrmann T., Geralt M., Wuthrich K. (2007). Novel beta-barrel fold in the nuclear magnetic resonance structure of the replicase nonstructural protein 1 from the severe acute respiratory syndrome coronavirus. J. Virol. 81, 3151–3161. doi: 10.1128/JVI.01939-06, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

