IL-27 Derived From Macrophages Facilitates IL-15 Production and T Cell Maintenance Following Allergic Hypersensitivity Responses
- PMID: 34659203
- PMCID: PMC8515907
- DOI: 10.3389/fimmu.2021.713304
IL-27 Derived From Macrophages Facilitates IL-15 Production and T Cell Maintenance Following Allergic Hypersensitivity Responses
Abstract
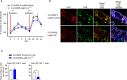
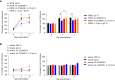
Crosstalk between T cells, dendritic cells, and macrophages in temporal leukocyte clusters within barrier tissues provides a new concept for T cell activation in the skin. Activated T cells from these leukocyte clusters play critical roles in the efferent phase of allergic contact hypersensitivity (CHS). However, the cytokines driving maintenance and survival of pathogenic T cells during and following CHS remain mostly unknown. Upon epicutaneous allergen challenge, we here report that macrophages produce IL-27 which then induces IL-15 production from epidermal keratinocytes and dermal myeloid cells within leukocyte clusters. In agreement with the known role of IL-15 as a T cell survival factor and growth cytokine, this signaling axis enhances BCL2 and survival of skin T cells. Genetic depletion or pharmacological blockade of IL-27 in CHS mice leads to abrogated epidermal IL-15 production resulting in a decrease in BCL2 expression in T cells and a decline in dermal CD8+ T cells and T cell cluster numbers. These findings suggest that the IL-27 pathway is an important cytokine for regulating cutaneous T cell immunity.
Keywords: BCL2; CD172a; IL-15; IL-27; STAT1; contact hypersensitivity; dermal leukocyte cluster; human allergic contact dermatitis.
Copyright © 2021 Suwanpradid, Lee, Hoang, Kwock, Floyd, Smith, Yin, Atwater, Rajagopal, Kedl, Corcoran, Zhang and MacLeod.
Conflict of interest statement
Author AM has received funding from Silab, Inc. and has consulted for the company. AM also served on the reviewer committee for the LEO foundation and the Triangle Community Foundation. Author AA received a Pfizer Independent Grant for Learning and Change and has consulted for Henkel. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

