Diagnostic Vaccination in Clinical Practice
- PMID: 34659207
- PMCID: PMC8514775
- DOI: 10.3389/fimmu.2021.717873
Diagnostic Vaccination in Clinical Practice
Abstract
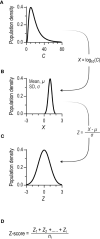
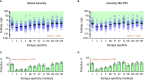
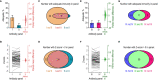
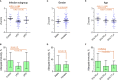
Testing the antibody response to vaccination (diagnostic vaccination) is crucial in the clinical evaluation of primary immunodeficiency diseases. Guidelines from the American Academy of Allergy, Asthma & Immunology (AAAAI) provide detailed recommendations for diagnostic vaccination with pure pneumococcal polysaccharide vaccines (PPV). However, the degree of compliance with these guidelines and the utility of the guidelines in actual practice are undescribed. To address this, we systematically evaluated diagnostic vaccination in adult patients with suspected primary immunodeficiency diseases in a single tertiary center from 2011 to 2016 (n = 229). We found that full compliance with the AAAAI guidelines was achieved for only 39 patients (17%), suggesting that the guidelines are not easy to follow. Worse, interpretation according to the guidelines was heavily influenced by which serotype-specific antibodies that were used for the evaluation. We found that the arbitrary choices of serotype-specific antibodies could change the fraction of patients deemed to have 'adequate immunity' by a factor of four, exposing an inherent flaw in the guidelines. The flaw relates to dichotomous principles for data interpretation under the AAAAI guidelines. We therefore propose a revised protocol for diagnostic vaccination limited to PPV vaccination, subsequent antibody measurements, and data interpretation using Z-scores. The Z-score compiles multiple individual antibody levels, adjusted for different weighting, into one single continuous variable for each patient. In contrast to interpretation according to the AAAAI guidelines, the Z-scores were robust to variations in the choice of serotype-specific antibodies used for interpretation. Moreover, Z-scores revealed reduced immunity after vaccination in the patients with recurrent pneumonia (a typical symptom of antibody deficiency) compared with control patients. Assessment according to the AAAAI guidelines failed to detect this difference. We conclude that our simplified protocol and interpretation with Z-scores provides more robust clinical results and may enhance the value of diagnostic vaccination.
Keywords: antibody deficiency; clinical guidelines; diagnostic vaccination; pneumococcal vaccines; primary immunodeficiency; vaccination; z-score.
Copyright © 2021 Hansen, Söderström, Jørgensen, Larsen, Petersen and Bernth Jensen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Seidel MG, Kindle G, Gathmann B, Quinti I, Buckland M, van Montfrans J, et al. . The European Society for Immunodeficiencies (ESID) Registry Working Definitions for the Clinical Diagnosis of Inborn Errors of Immunity. J Allergy Clin Immunol Pract (2019) 7(6):1763–70. doi: 10.1016/j.jaip.2019.02.004 - DOI - PubMed
-
- (LASID) LAfI . Primary Immunodeficiency (2021). Available at: https://lasid.org/primary-immunodeficiency/.
-
- Winkelstein JA, Marino MC, Lederman HM, Jones SM, Sullivan K, Burks AW, et al. . X-Linked Agammaglobulinemia: Report on a United States Registry of 201 Patients. Medicine (2006) 85(4):193–202. doi: 10.1097/01.md.0000229482.27398.ad - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

