Poor CD4+ T Cell Immunogenicity Limits Humoral Immunity to P. falciparum Transmission-Blocking Candidate Pfs25 in Humans
- PMID: 34659219
- PMCID: PMC8515144
- DOI: 10.3389/fimmu.2021.732667
Poor CD4+ T Cell Immunogenicity Limits Humoral Immunity to P. falciparum Transmission-Blocking Candidate Pfs25 in Humans
Abstract
Plasmodium falciparum transmission-blocking vaccines (TBVs) targeting the Pfs25 antigen have shown promise in mice but the same efficacy has never been achieved in humans. We have previously published pre-clinical data related to a TBV candidate Pfs25-IMX313 encoded in viral vectors which was very promising and hence progressed to human clinical trials. The results from the clinical trial of this vaccine were very modest. Here we unravel why, contrary to mice, this vaccine has failed to induce robust antibody (Ab) titres in humans to elicit transmission-blocking activity. We examined Pfs25-specific B cell and T follicular helper (Tfh) cell responses in mice and humans after vaccination with Pfs25-IMX313 encoded by replication-deficient chimpanzee adenovirus serotype 63 (ChAd63) and the attenuated orthopoxvirus modified vaccinia virus Ankara (MVA) delivered in the heterologous prime-boost regimen via intramuscular route. We found that after vaccination, the Pfs25-IMX313 was immunologically suboptimal in humans compared to mice in terms of serum Ab production and antigen-specific B, CD4+ and Tfh cell responses. We identified that the key determinant for the poor anti-Pfs25 Ab formation in humans was the lack of CD4+ T cell recognition of Pfs25-IMX313 derived peptide epitopes. This is supported by correlations established between the ratio of proliferated antigen-specific CD4+/Tfh-like T cells, CXCL13 sera levels, and the corresponding numbers of circulating Pfs25-specific memory B cells, that consequently reflected on antigen-specific IgG sera levels. These correlations can inform the design of next-generation Pfs25-based vaccines for robust and durable blocking of malaria transmission.
Keywords: CD4+; P. falciparum; Pfs25; transmission blocking; vaccine.
Copyright © 2021 Zaric, Marini, Nielsen, Gupta, Mekhaiel, Pham, Elias, Taylor, de Graaf, Payne, Li, Silk, Williams, Hill, Long, Miura and Biswas.
Conflict of interest statement
AH is named inventor on patent applications covering malaria vaccines and immunization regimens. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




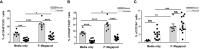


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

