Soybean Cyst Nematodes Influence Aboveground Plant Volatile Signals Prior to Symptom Development
- PMID: 34659318
- PMCID: PMC8513716
- DOI: 10.3389/fpls.2021.749014
Soybean Cyst Nematodes Influence Aboveground Plant Volatile Signals Prior to Symptom Development
Abstract
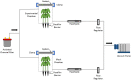
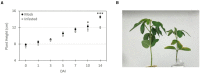
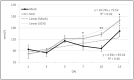
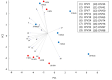
Soybean cyst nematode (SCN), Heterodera glycines, is one of the most destructive soybean pests worldwide. Unlike many diseases, SCN doesn't show above ground evidence of disease until several weeks after infestation. Knowledge of Volatile Organic Compounds (VOCs) related to pests and pathogens of foliar tissue is extensive, however, information related to above ground VOCs in response to root damage is lacking. In temporal studies, gas chromatography-mass spectrometry analysis of VOCs from the foliar tissues of SCN infested plants yielded 107 VOCs, referred to as Common Plant Volatiles (CPVs), 33 with confirmed identities. Plants showed no significant stunting until 10 days after infestation. Total CPVs increased over time and were significantly higher from SCN infested plants compared to mock infested plants post 7 days after infestation (DAI). Hierarchical clustering analysis of expression ratios (SCN: Mock) across all time points revealed 5 groups, with the largest group containing VOCs elevated in response to SCN infestation. Linear projection of Principal Component Analysis clearly separated SCN infested from mock infested plants at time points 5, 7, 10 and 14 DAI. Elevated Styrene (CPV11), D-Limonene (CPV32), Tetradecane (CPV65), 2,6-Di-T-butyl-4-methylene-2,5-cyclohexadiene-1-one (CPV74), Butylated Hydroxytoluene (CPV76) and suppressed Ethylhexyl benzoate (CPV87) levels, were associated with SCN infestation prior to stunting. Our findings demonstrate that SCN infestation elevates the release of certain VOCs from foliage and that some are evident prior to symptom development. VOCs associated with SCN infestations prior to symptom development may be valuable for innovative diagnostic approaches.
Keywords: GC-MS; VOCs; early disease detection; soybean; soybean cyst nematode.
Copyright © 2021 Constantino, Oh, Şennik, Andersen, Warden, Oralkan and Dean.
Conflict of interest statement
MW was employed by BASF Plant Science. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from BASF Plant Science. The funder had the following involvement in the study: MW contributed to plant propagation and infestation of plants with nematodes.
Figures



Similar articles
-
First Report of the Soybean Cyst Nematode, Heterodera glycines, on Soybean in Zhejiang, Eastern China.Plant Dis. 2009 Mar;93(3):319. doi: 10.1094/PDIS-93-3-0319B. Plant Dis. 2009. PMID: 30764206
-
Soybean cyst nematode detection and management: a review.Plant Methods. 2022 Sep 7;18(1):110. doi: 10.1186/s13007-022-00933-8. Plant Methods. 2022. PMID: 36071455 Free PMC article. Review.
-
Transcriptome profiling of interaction effects of soybean cyst nematodes and soybean aphids on soybean.Sci Data. 2019 Jul 24;6(1):133. doi: 10.1038/s41597-019-0140-4. Sci Data. 2019. PMID: 31341170 Free PMC article.
-
First Report of Soybean Cyst Nematode (Heterodera glycines) on Tobacco in Henan, Central China.Plant Dis. 2013 Jun;97(6):852. doi: 10.1094/PDIS-10-12-0926-PDN. Plant Dis. 2013. PMID: 30722601
-
Molecular Basis of Soybean Resistance to Soybean Aphids and Soybean Cyst Nematodes.Plants (Basel). 2019 Sep 26;8(10):374. doi: 10.3390/plants8100374. Plants (Basel). 2019. PMID: 31561499 Free PMC article. Review.
Cited by
-
Biochemical analysis of the TPS-a subfamily in Medicago truncatula.Front Plant Sci. 2024 Feb 15;15:1349009. doi: 10.3389/fpls.2024.1349009. eCollection 2024. Front Plant Sci. 2024. PMID: 38425791 Free PMC article.
References
-
- Ansari R. A., Rizvi R., Mahmood I. (2020). Management of Phytonematodes: Recent Advances and Future Challenges, Management of Phytonematodes: Recent Advances and Future Challenges. Singapore: Springer.
-
- Arpaia S., de Cristofaro A., Guerrieri E., Bossi S., Cellini F., Leo G. M. D., et al. . (2011). Foraging activity of bumblebees (Bombus terrestris L.) on Bt-expressing eggplants. Arthropod-Plant Interact. 5, 255–261. 10.1007/s11829-011-9144-5 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

