Traditional Chinese Medicine Yang-Gan-Wan Alleviated Experimental Hepatic Damage by Inhibiting Oxidation, Inflammation, and Apoptosis in Cell and Mouse Models
- PMID: 34659428
- PMCID: PMC8514921
- DOI: 10.1155/2021/2556352
Traditional Chinese Medicine Yang-Gan-Wan Alleviated Experimental Hepatic Damage by Inhibiting Oxidation, Inflammation, and Apoptosis in Cell and Mouse Models
Abstract
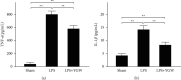
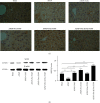
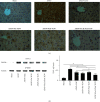
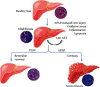
A hepatoprotective medicine, Yang-Gan-Wan (YGW), was used to treat hepatic damage in cell and mouse models. We performed a 1,1-diphenyl-2- picrylhydrazyl (DPPH) assay and found that YGW exhibited a significantly high free radical scavenging ability. Furthermore, the results of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay revealed that YGW treatment could alleviate lipopolysaccharide (LPS)-induced damage in Kupffer cells (liver macrophages). Enzyme-linked immunosorbent assay results demonstrated that YGW treatment could alleviate LPS-induced inflammation in Kupffer cells by inhibiting the expression of tumor necrosis factor (TNF)-α and interleukin (IL)-1β. By quantifying the serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), we found that YGW treatment could alleviate hepatic damage and improve immunity in acetaminophen- (APAP-) treated mice by inhibiting the expression of ALT and AST. The findings of hematoxylin and eosin and Masson's trichrome staining indicated that YGW treatment could alleviate hepatic damage and reduce collagen fiber formation in the liver tissue of APAP-treated mice. Furthermore, immunohistochemistry staining and Western blot results showed that YGW treatment could alleviate oxidative stress, inflammation, and apoptosis in the liver tissue of APAP-treated mice by enhancing superoxide dismutase 2 (SOD2) expression but inhibiting TNF-α and caspase 3 expression. Our results suggest that YGW treatment exerted hepatoprotective effects on LPS-treated Kupffer cells and APAP-treated mice by inhibiting oxidation, inflammation, and apoptosis.
Copyright © 2021 Chia-Wen Yeh et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures







References
-
- Sakaida I., Matsumura Y., Akiyama S., Hayashi K., Ishige A., Okita K. Herbal medicine Sho-saiko-to (TJ-9) prevent liver fibrosis and enzyme-altered lesions in rat liver cirrhosis induced by a choline-deficient l-amino acid-defined diet. Journal of Hepatology . 1998;28(2):298–306. doi: 10.1016/0168-8278(88)80017-5. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

