Long non-coding RNA NEAT1 contributes to lipopolysaccharide-induced inflammation and apoptosis of human middle ear epithelial cells via regulating the miR-301b-3p/TLR4 axis
- PMID: 34659506
- PMCID: PMC8515508
- DOI: 10.3892/etm.2021.10795
Long non-coding RNA NEAT1 contributes to lipopolysaccharide-induced inflammation and apoptosis of human middle ear epithelial cells via regulating the miR-301b-3p/TLR4 axis
Abstract
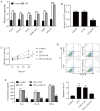
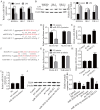
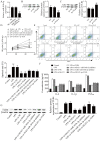
Acute otitis media (AOM) is a common infectious disease in children that is accompanied by signs and symptoms of middle ear inflammation and infection. Previous studies have shown that the long non-coding (lnc)RNA nuclear-enriched abundant transcript 1(NEAT1) participates in various inflammatory conditions and plays an important regulatory role. The focus of the present study was the biological function of NEAT1 and underlying molecular mechanism in lipopolysaccharide (LPS)-induced human middle ear epithelial cells (HMEECs). The expression of NEAT1, miR-301b-3p and toll-like receptor 4 (TLR4) protein were determined by reverse transcription-quantitative PCR and western blot assays, respectively. Dual-luciferase reporter assay was performed to investigate the combination of miR-301b-3p and NEAT1 or TLR4. In addition, cell viability, apoptosis and the levels of pro-inflammatory factors (IL-1β, TNF-α and IL-6) were measured by Cell Counting Kit-8 assay, flow cytometry and ELISA, respectively. Cell viability was significantly decreased, whereas apoptosis and inflammation were increased in LPS-stimulated HMEECs. Functional analyses demonstrated that NEAT1 was upregulated following LPS treatment, whereas knockdown of NEAT1 significantly increased cell viability and alleviated apoptosis and inflammation. Mechanistically, NEAT1 directly bound to and negatively regulated miR-301b-3p expression, whereas miR-301b-3p inhibitors abolished the inhibitory effect of NEAT1 knockdown on cell apoptosis and inflammation. As a target of miR-301b-3p, TLR4 was regulated by NEAT1 and miR-301b-3p. TLR4 overexpression alleviated NEAT1 silencing-induced inflammatory suppression. Rescue experiments demonstrated that NEAT1 promoted TLR4 expression by inhibiting miR-301b-3p. Collectively, the results of the present study suggested that NEAT1 may attenuate LPS-induced inflammation and apoptosis in HMEECs by modulating the miR-301b-3p/TLR4 axis, and may provide a new therapeutic target for the clinical treatment of AOM.
Keywords: acute otitis media; human middle ear epithelial cells; miR-301b-3p; nuclear-enriched abundant transcript 1; toll-like receptor 4.
Copyright: © Liu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
