Organocatalytic asymmetric formal oxidative coupling for the construction of all-aryl quaternary stereocenters
- PMID: 34659717
- PMCID: PMC8442720
- DOI: 10.1039/d1sc03324g
Organocatalytic asymmetric formal oxidative coupling for the construction of all-aryl quaternary stereocenters
Abstract
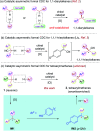
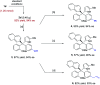
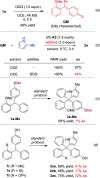
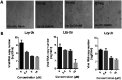
A new catalytic asymmetric formal cross dehydrogenative coupling process for the construction of all-aryl quaternary stereocenters is disclosed, which provides access to rarely explored chiral tetraarylmethanes with excellent enantioselectivity. The suitable oxidation conditions and the hydrogen-bond-based organocatalysis have enabled efficient intermolecular C-C bond formation in an overwhelmingly crowded environment under mild conditions. para-Quinone methides bearing an ortho-directing group serve as the key intermediate. The precise loading of DDQ is critical to the high enantioselectivity. The chiral products have also been demonstrated as promising antiviral agents.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
-
Reviews of CDC reactions:
- Li C.-J. Acc. Chem. Res. 2009;42:335–344. doi: 10.1021/ar800164n. - DOI - PubMed
- Yeung C. S. Dong V. M. Chem. Rev. 2011;111:1215–1292. doi: 10.1021/cr100280d. - DOI - PubMed
- Girard S. A. Knauber T. Li C.-J. Angew. Chem., Int. Ed. 2014;53:74–100. doi: 10.1002/anie.201304268. - DOI - PubMed
-
-
-
Selected examples of catalytic asymmetric formal CDCs for the synthesis of 1,1-diarylalkanes:
- Wu H. He Y.-P. Xu L. Zhang D.-Y. Gong L.-Z. Angew. Chem., Int. Ed. 2014;53:3466–3469. doi: 10.1002/anie.201309967. - DOI - PubMed
- Guo C. Song J. Luo S.-W. Gong L.-Z. Angew. Chem., Int. Ed. 2010;49:5558–5562. doi: 10.1002/anie.201002108. - DOI - PubMed
- Larionov E. Mastandrea M. M. Pericàs M. A. ACS Catal. 2017;7:7008–7013. doi: 10.1021/acscatal.7b02659. - DOI
- Benfatti F. Capdevila M. G. Zoli L. Benedetto E. Cozzi P. G. Chem. Commun. 2009:5919–5921. doi: 10.1039/B910185C. - DOI - PubMed
-
-
-
For reviews (a and b) and selected examples (c–j) of asymmetric synthesis of chiral triarylmethanes:
- Nambo M. Crudden C. M. ACS Catal. 2015;5:4734–4742. doi: 10.1021/acscatal.5b00909. - DOI
- Kshatriya R. Jejurkar V. P. Saha S. Eur. J. Org. Chem. 2019:3818–3841. doi: 10.1002/ejoc.201900465. - DOI
- Mondal S. Roy D. Panda G. ChemCatChem. 2018;10:1941–1967. doi: 10.1002/cctc.201701601. - DOI
- Matthew S. C. Glasspoole B. W. Eisenberger P. Crudden C. M. J. Am. Chem. Soc. 2014;136:5828–5831. doi: 10.1021/ja412159g. - DOI - PubMed
- Huang Y. Hayashi T. J. Am. Chem. Soc. 2015;137:7556–7559. doi: 10.1021/jacs.5b03277. - DOI - PubMed
- Pan T. Shi P. Chen B. Zhou D.-G. Zeng Y.-L. Chu W.-D. He L. Liu Q.-Z. Fan C.-A. Org. Lett. 2019;21:6397–6402. doi: 10.1021/acs.orglett.9b02308. - DOI - PubMed
- Shi B.-F. Maugel N. Zhang Y.-H. Yu J.-Q. Angew. Chem., Int. Ed. 2008;47:4882–4886. doi: 10.1002/anie.200801030. - DOI - PubMed
- Lou Y. Cao P. Jia T. Zhang Y. Wang M. Liao J. Angew. Chem., Int. Ed. 2015;54:12134–12138. doi: 10.1002/anie.201505926. - DOI - PubMed
- Saha S. Alamsetti S. K. Schneider C. Chem. Commun. 2015;51:1461–1464. doi: 10.1039/C4CC08559K. - DOI - PubMed
- Kim J. H. Greßies S. Boultadakis-Arapinis M. Daniliuc C. Glorius F. ACS Catal. 2016;6:7652–7656. doi: 10.1021/acscatal.6b02392. - DOI
- Franklin M. R. Biochem. Pharmacol. 1993;46:683–689. doi: 10.1016/0006-2952(93)90555-B. - DOI - PubMed
-
-
-
Construction of quaternary stereocenters is a longstanding challenge in organic synthesis, for selected reviews:
- Quasdorf K. Overman W. L. E. Nature. 2014;516:181–191. doi: 10.1038/nature14007. - DOI - PMC - PubMed
- Prakash J. Marek I. Chem. Commun. 2011;47:4593–4623. doi: 10.1039/C0CC05222A. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources
Other Literature Sources

