TIM-3 in Leukemia; Immune Response and Beyond
- PMID: 34660319
- PMCID: PMC8514831
- DOI: 10.3389/fonc.2021.753677
TIM-3 in Leukemia; Immune Response and Beyond
Abstract
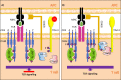
T cell immunoglobulin and mucin domain 3 (TIM-3) expression on malignant cells has been reported in some leukemias. In myelodysplastic syndrome (MDS), increased TIM-3 expression on TH1 cells, regulatory T cells, CD8+ T cells, and hematopoietic stem cells (HSCs), which play a role in the proliferation of blasts and induction of immune escape, has been reported. In AML, several studies have reported overexpression of TIM-3 on leukemia stem cells (LSCs) but not on healthy HSCs. Overexpression of TIM-3 on exhausted CD4+ and CD8+ T cells and leukemic cells in CML, ALL, and CLL patients could be a prognostic risk factor for poor therapeutic response and relapse in patients. Currently, several TIM-3 inhibitors are used in clinical trials for leukemias, and some have shown encouraging response rates for MDS and AML treatment. For AML immunotherapy, blockade TIM-3 may have dual effects: directly inhibiting AML cell proliferation and restoring T cell function. However, blockade of PD-1 and TIM-3 fails to restore the function of exhausted CD8+ T cells in the early clinical stages of CLL, indicating that the effects of TIM-3 blockade may be different in AML and other leukemias. Thus, further studies are required to evaluate the efficacy of TIM-3 inhibitors in different types and stages of leukemia. In this review, we summarize the biological functions of TIM-3 and its contribution as it relates to leukemias. We also discuss the effects of TIM-3 blockade in hematological malignancies and clinical trials of TIM-3 for leukemia therapy.
Keywords: TIM-3; acute lymphoblastic leukemia; acute myeloid leukemia; chronic lymphoblastic leukemia; chronic myeloid leukemia; myelodysplastic syndrome.
Copyright © 2021 Rezaei, Tan, Zeng, Li and Ganjalikhani-Hakemi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Higher PD-1/Tim-3 expression on IFN-γ+ T cells is associated with poor prognosis in patients with acute myeloid leukemia.Cancer Biol Ther. 2023 Dec 31;24(1):2278229. doi: 10.1080/15384047.2023.2278229. Epub 2023 Nov 14. Cancer Biol Ther. 2023. PMID: 37962843 Free PMC article.
-
Increased TOX expression concurrent with PD-1, Tim-3, and CD244 expression in T cells from patients with acute myeloid leukemia.Cytometry B Clin Cytom. 2022 Mar;102(2):143-152. doi: 10.1002/cyto.b.22049. Epub 2021 Dec 16. Cytometry B Clin Cytom. 2022. PMID: 34913594
-
Blockade of PD-1 and TIM-3 immune checkpoints fails to restore the function of exhausted CD8+ T cells in early clinical stages of chronic lymphocytic leukemia.Immunol Res. 2020 Oct;68(5):269-279. doi: 10.1007/s12026-020-09146-4. Immunol Res. 2020. PMID: 32710227
-
Identification of TIM-3 as a Leukemic Stem Cell Surface Molecule in Primary Acute Myeloid Leukemia.Oncology. 2015;89 Suppl 1:28-32. doi: 10.1159/000431062. Epub 2015 Nov 10. Oncology. 2015. PMID: 26551150 Review.
-
TIM-3 in normal and malignant hematopoiesis: Structure, function, and signaling pathways.Cancer Sci. 2021 Sep;112(9):3419-3426. doi: 10.1111/cas.15042. Epub 2021 Jul 14. Cancer Sci. 2021. PMID: 34159709 Free PMC article. Review.
Cited by
-
Design and Evaluation of TIM-3-CD28 Checkpoint Fusion Proteins to Improve Anti-CD19 CAR T-Cell Function.Front Immunol. 2022 Apr 6;13:845499. doi: 10.3389/fimmu.2022.845499. eCollection 2022. Front Immunol. 2022. PMID: 35464394 Free PMC article.
-
TIM3, a human acute myeloid leukemia stem cell marker, does not enrich for leukemia-initiating stem cells in B-cell acute lymphoblastic leukemia.Haematologica. 2023 Aug 1;108(8):2229-2233. doi: 10.3324/haematol.2022.282394. Haematologica. 2023. PMID: 36655434 Free PMC article. No abstract available.
-
The role of bone marrow microenvironment (BMM) cells in acute myeloid leukemia (AML) progression: immune checkpoints, metabolic checkpoints, and signaling pathways.Cell Commun Signal. 2023 Sep 21;21(1):252. doi: 10.1186/s12964-023-01282-2. Cell Commun Signal. 2023. PMID: 37735675 Free PMC article. Review.
-
Updates in novel immunotherapeutic strategies for relapsed/refractory AML.Front Oncol. 2024 Dec 4;14:1374963. doi: 10.3389/fonc.2024.1374963. eCollection 2024. Front Oncol. 2024. PMID: 39697225 Free PMC article. Review.
-
Bio-functional hydrogel coated membranes to decrease T-cell exhaustion in manufacturing of CAR T-cells.Front Immunol. 2025 Jun 27;16:1513148. doi: 10.3389/fimmu.2025.1513148. eCollection 2025. Front Immunol. 2025. PMID: 40655138 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

