Quantitative Analysis of Temporal Bone Density and Thickness for Robotic Ear Surgery
- PMID: 34660681
- PMCID: PMC8514837
- DOI: 10.3389/fsurg.2021.740008
Quantitative Analysis of Temporal Bone Density and Thickness for Robotic Ear Surgery
Abstract
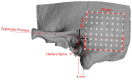
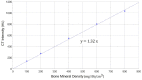
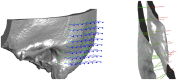
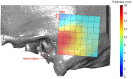
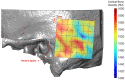
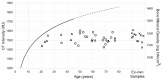
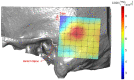
Background and Objective: Quantitative assessment of bone density and thickness in computed-tomography images offers great potential for preoperative planning procedures in robotic ear surgery. Methods: We retrospectively analyzed computed-tomography scans of subjects undergoing cochlear implantation (N = 39). In addition, scans of Thiel-fixated ex-vivo specimens were analyzed (N = 15). To estimate bone mineral density, quantitative computed-tomography data were obtained using a calibration phantom. The temporal bone thickness and cortical bone density were systematically assessed at retroauricular positions using an automated algorithm referenced by an anatomy-based coordinate system. Two indices are proposed to include information of bone density and thickness for the preoperative assessment of safe screw positions (Screw Implantation Safety Index, SISI) and mass distribution (Column Density Index, CODI). Linear mixed-effects models were used to assess the effects of age, gender, ear side and position on bone thickness, cortical bone density and the distribution of the indices. Results: Age, gender, and ear side only had negligible effects on temporal bone thickness and cortical bone density. The average radiodensity of cortical bone was 1,511 Hounsfield units, corresponding to a bone mineral density of 1,145 mg HA/cm3. Temporal bone thickness and cortical bone density depend on the distance from Henle's spine in posterior direction. Moreover, safe screw placement locations can be identified by computation of the SISI distribution. A local maximum in mass distribution was observed posteriorly to the supramastoid crest. Conclusions: We provide quantitative information about temporal bone density and thickness for applications in robotic and computer-assisted ear surgery. The proposed preoperative indices (SISI and CODI) can be applied to patient-specific cases to identify optimal regions with respect to bone density and thickness for safe screw placement and effective implant positioning.
Keywords: BAHA; bone conduction implants; bone mineral density; bone thickness; calibrated Hounsfield units; quantitative computed-tomography; screw safety.
Copyright © 2021 Talon, Visini, Wagner, Caversaccio and Wimmer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor PH declared a past co-authorship with the authors MC and WW.
Figures



References
LinkOut - more resources
Full Text Sources

