Effects of Heparin and Bivalirudin on Thrombin-Induced Platelet Activation: Differential Modulation of PAR Signaling Drives Divergent Prothrombotic Responses
- PMID: 34660719
- PMCID: PMC8511449
- DOI: 10.3389/fcvm.2021.717835
Effects of Heparin and Bivalirudin on Thrombin-Induced Platelet Activation: Differential Modulation of PAR Signaling Drives Divergent Prothrombotic Responses
Abstract
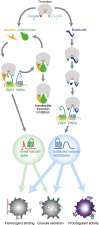
Heparin and bivalirudin are widely used as anticoagulants in the setting of acute thrombosis. In this study, we investigated how these drugs affect the ability of thrombin to generate a prothrombotic platelet response via activation of the protease-activated receptors (PARs) 1 and 4. We examined the effects of heparin/antithrombin and bivalirudin on PAR1- and PAR4-mediated intracellular calcium mobilization, aggregation, α-granule release, and procoagulant membrane exposure in platelets exposed to thrombin concentrations likely to be encountered in the thrombus microenvironment during thrombosis. At physiological antithrombin levels, heparin treatment resulted in complete and sustained inhibition of thrombin-induced PAR4-mediated platelet activation, but transient PAR1 signaling was sufficient to elicit significant α-granule release and platelet aggregation. In contrast, bivalirudin treatment resulted in rapid and profound inhibition of signaling from both PAR receptors, followed by a delayed phase of PAR4-mediated platelet activation, resulting in a robust prothrombotic response. Combination treatment with bivalirudin and subtherapeutic concentrations of heparin completely inhibited the residual platelet activation observed with single drug treatment at all time-points. Our results show that heparin and bivalirudin have different and complementary inhibitory effects on the activation of PAR1 and PAR4 by thrombin.
Keywords: PAR1; PAR4; bivalirudin; heparin; thrombin.
Copyright © 2021 Lund, Macwan, Tunströmer, Lindahl and Boknäs.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

