Vasorelaxant-Mediated Antihypertensive Effect of the Leaf Aqueous Extract from Stephania abyssinica (Dillon & A. Rich) Walp (Menispermaceae) in Rat
- PMID: 34660790
- PMCID: PMC8519676
- DOI: 10.1155/2021/4730341
Vasorelaxant-Mediated Antihypertensive Effect of the Leaf Aqueous Extract from Stephania abyssinica (Dillon & A. Rich) Walp (Menispermaceae) in Rat
Abstract
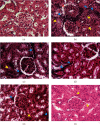
Stephania abyssinica is a medicinal plant used in Cameroon alternative medicine to treat arterial hypertension (AHT). Previous in vitro studies demonstrated the endothelium nitric oxide-independent vasorelaxant property of the aqueous extract from Stephania abyssinica (AESA). But its effect on AHT is unknown. The present study was undertaken to explore other vasorelaxant mechanisms and to determine the antihypertensive effects of AESA in male Wistar rats. Phytochemical analysis of AESA was carried out using the liquid chromatography-mass spectrometry (LC-MS) method. The vasorelaxant effects of AESA (1-1000 μg/mL) were studied on rat isolated thoracic aorta rings, in the absence or presence of indomethacin (10 μM) or methylene blue (10 μM). The inhibitory effect of AESA on phenylephrine (PE, 10 μM) or KCl- (60 mM) induced contraction as well as the intracellular calcium release was also evaluated. The in vivo antihypertensive activity of AESA (43, 86, or 172 mg/kg/day) or captopril (20 mg/kg/day) administered orally was assessed in L-NAME- (40 mg/kg/day) treated rats. Blood pressure and heart rate (HR) were measured at the end of each week while serum or urinary nitric oxide (NO), creatinine, and glomerular filtration rate (GFR) were determined at the end of the 6 weeks of treatment, as well as histological analysis of the heart and the kidney. The LC-MS profiling of AESA identified 9 compounds including 7 alkaloids. AESA produced a concentration-dependent relaxation on contraction induced either by PE and KCl, which was significantly reduced in endothelium-denuded vessels, as well as in vessels pretreated with indomethacin and methylene blue. Moreover, AESA inhibited the intracellular Ca2+ release-induced contraction. In vivo, AESA reduced the AHT, heart rate (HR), and ventricular hypertrophy and increased serum NO, urine creatinine, and GFR. AESA also ameliorated heart and kidney lesions as compared to the L-NAME group. These findings supported the use of AESA as a potential antihypertensive drug.
Copyright © 2021 Chamberlin Fodem et al.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures







References
-
- Institute for Health Metrics and Evaluation. High systolic blood pressure-level 2 risk . IHME, University of Washington; 2019. June 2021, http://www.healthdata.org/results/gbd_summaries/2019/high-systolic-blood....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

