Bispecific Antibodies in Multiple Myeloma: Present and Future
- PMID: 34661161
- PMCID: PMC8510808
- DOI: 10.1158/2643-3230.BCD-21-0028
Bispecific Antibodies in Multiple Myeloma: Present and Future
Abstract
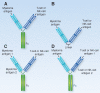
Despite many recent advances in therapy, there is still no plateau in overall survival curves in multiple myeloma. Bispecific antibodies are a novel immunotherapeutic approach designed to bind antigens on malignant plasma cells and cytotoxic immune effector cells. Early-phase clinical trials targeting B-cell maturation antigen (BCMA), GPRC5D, and FcRH5 have demonstrated a favorable safety profile, with mainly low-grade cytokine release syndrome, cytopenias, and infections. Although dose escalation is ongoing in several studies, early efficacy data show response rates in the most active dose cohorts between 61% and 83% with many deep responses; however, durability remains to be established. Further clinical trial data are eagerly anticipated.
Significance: Overall survival of triple-class refractory multiple myeloma remains poor. Bispecific antibodies are a novel immunotherapeutic modality with a favorable safety profile and impressive preliminary efficacy in heavily treated patients. Although more data are needed, bispecifics will likely become an integral part of the multiple myeloma treatment paradigm in the near future. Studies in earlier lines of therapy and in combination with other active anti-multiple myeloma agents will help further define the role of bispecifics in multiple myeloma.
©2021 American Association for Cancer Research.
Figures

References
-
- Kontermann RE, Brinkmann U.Bispecific antibodies. Drug Discov Today 2015;20:838–47. - PubMed
-
- Ellerman D.Bispecific T-cell engagers: towards understanding variables influencing the in vitro potency and tumor selectivity and their modulation to enhance their efficacy and safety. Methods 2019;154:102–17. - PubMed
-
- Adams GP, Schier R, McCall AM, Simmons HH, Horak EM, Alpaugh RK, et al. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res 2001;61:4750–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

