Prophylactic or very early initiation of continuous positive airway pressure (CPAP) for preterm infants
- PMID: 34661278
- PMCID: PMC8521644
- DOI: 10.1002/14651858.CD001243.pub4
Prophylactic or very early initiation of continuous positive airway pressure (CPAP) for preterm infants
Abstract
Background: Cohort studies have suggested that nasal continuous positive airway pressure (CPAP) starting in the immediate postnatal period before the onset of respiratory disease (prophylactic CPAP) may be beneficial in reducing the need for intubation and intermittent positive pressure ventilation (IPPV), and in preventing bronchopulmonary dysplasia (BPD), in preterm or low birth weight infants.
Objectives: To determine if prophylactic nasal CPAP (started within the first 15 minutes) or very early nasal CPAP regardless of respiratory status (started within the first hour of life), reduces the use of mechanical ventilation and the incidence of bronchopulmonary dysplasia without any adverse effects in preterm infants.
Search methods: A comprehensive search was run on 6 November 2020 in the Cochrane Central Register of Controlled Trials (CENTRAL via CRS Web) and MEDLINE via Ovid. We also searched the reference lists of retrieved studies.
Selection criteria: We included all randomised controlled trials (RCTs) and quasi-RCTs in preterm infants (under 37 weeks of gestation). We included trials if they compared prophylactic nasal CPAP (started within the first 15 minutes) or very early nasal CPAP (started within the first hour of life) in infants with minimal signs of respiratory distress with 'supportive care', such as supplemental oxygen therapy, standard nasal cannula, or mechanical ventilation. We excluded studies where prophylactic CPAP was compared with CPAP along with co-interventions.
Data collection and analysis: We used the standard methods of Cochrane Neonatal, including independent study selection, assessment of trial quality, and extraction of data by two review authors.
Main results: We included eight trials (seven from the previous version of the review and one new study), recruiting 3201 babies, in the meta-analysis. Four trials, involving 765 babies, compared CPAP with supportive care, and three trials (2364 babies) compared CPAP with mechanical ventilation. One trial (72 babies) compared prophylactic CPAP with very early CPAP. Apart from a lack of blinding of the intervention, we judged seven studies to have a low risk of bias. However, one study had a high risk of selection bias. Prophylactic or very early CPAP compared to supportive care There may be a reduction in failed treatment (risk ratio (RR) 0.6, 95% confidence interval (CI) 0.49 to 0.74; risk difference (RD) -0.16, 95% CI -0.34 to 0.02; 4 studies, 765 infants; very low certainty evidence). CPAP possibly reduces BPD at 36 weeks (RR 0.76, 95% CI 0.51 to 1.14; 3 studies, 683 infants, moderate certainty evidence); there may be little or no difference in death (RR 1.04, 95% CI 0.56 to 1.93; 4 studies, 765 infants; moderate certainty evidence). Prophylactic CPAP may reduce the composite outcome of death or BPD (RR 0.69, 95% CI 0.40 to 1.19; 1 study, 256 infants; low certainty evidence). There may be no difference in pulmonary air leak (pneumothorax) (RR 0.75, 95% CI 0.35 to 1.16; 3 studies, 568 infants; low certainty evidence), or intraventricular haemorrhage (IVH) Grade 3 or 4 (RR 0.96, 95% CI 0.39 to 2.37; 2 studies, 486 infants; moderate certainty evidence). Neurodevelopmental impairment was not reported in any of the studies. Prophylactic or very early CPAP compared to mechanical ventilation There was probably a reduction in the incidence of BPD at 36 weeks (RR 0.89, 95% CI 0.8 to 0.99; RD -0.04, 95% CI -0.08 to 0.00; 3 studies, 2150 infants; moderate certainty evidence); and death or BPD (RR 0.89, 95% CI 0.81 to 0.97; RD -0.05, 95% CI -0.09 to 0.01; 3 studies, 2358 infants; moderate certainty evidence). There was also probably a reduction in the need for mechanical ventilation (failed treatment) (RR 0.49, 95% CI 0.45 to 0.54; RD -0.50, 95% CI -0.54 to -0.45; 2 studies, 1042 infants; moderate certainty evidence). There was probably a reduction in the incidence of death (RR 0.82, 95% CI 0.66 to 1.03; 3 studies, 2358 infants; moderate certainty evidence); pulmonary air leak (pneumothorax) (RR 1.24, 95% CI 0.91 to 1.69; 3 studies, 2357 infants; low certainty evidence); and IVH Grade 3 or 4 (RR 1.09, 95% CI 0.86 to 1.39; 3 studies, 2301 infants; moderate certainty evidence). One study in this comparison reported that there was probably little or no difference between the groups in the incidence of neurodevelopmental impairment at 18 to 22 months (RR 0.91, 95% CI 0.62 to 1.32; 976 infants; moderate certainty evidence). Prophylactic CPAP compared with very early CPAP There was one study in this comparison. We are very uncertain whether there is any difference in the incidence of BPD (RR 0.5, 95% CI 0.05 to 5.27; very low certainty evidence). The combined outcome of death and BPD was not reported, and failed treatment was reported but without data. There may have been little to no effect on death (RR 0.75, 95% CI 0.29 to1.94; 1 study, 72 infants; very low certainty evidence). Intraventricular haemorrhage Grade 3 or 4 and neurodevelopmental outcomes were not reported in this study. Pulmonary air leak (pneumothorax) was reported in this study, but there were no events in either group.
Authors' conclusions: For preterm and very preterm infants, there is insufficient evidence to evaluate prophylactic CPAP compared to oxygen therapy and other supportive care. When compared to mechanical ventilation, prophylactic nasal CPAP in very preterm infants reduces the incidence of BPD, the combined outcome of death and BPD, and mechanical ventilation. There is probably no difference in neurodevelopmental impairment at 18 to 22 months of age. When prophylactic CPAP is compared to early CPAP, we are very uncertain about whether there is any difference between prophylactic and very early CPAP. There is no information about the effect of prophylactic or very early CPAP in late preterm infants. There is one study awaiting classification.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Prema Subramaniam has declared no conflicts.
Jacqueline J Ho has worked as a Consultant Neonatologist for the Ministry of Health, Malaysia, as a Neonatal Unit Director.
Peter G Davis is a Neonatologist who receives project and salary support from the Australian National Health and Medical Research Council. He was involved in conducting an included study, the COIN trial (Morley 2008). This study was funded by the Australian National Health and Medical Research Council. JJH and PS independently extracted the data from the COIN study.
Figures
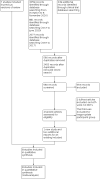
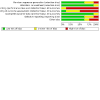











































Update of
-
Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants.Cochrane Database Syst Rev. 2016 Jun 14;(6):CD001243. doi: 10.1002/14651858.CD001243.pub3. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2021 Oct 18;10:CD001243. doi: 10.1002/14651858.CD001243.pub4. PMID: 27315509 Updated.
References
References to studies included in this review
Badiee 2013 {published data only}
-
- Badiee Z, Naseri F, Sadeghnia A. Early versus delayed initiation of nasal continuous positive airway pressure for treatment of respiratory distress syndrome in premature newborns: a randomized clinical trial. Advanced Biomedical Research 2013;2:4. [DOI: 10.4103/2277-9175.107965] [PMID: ] - DOI - PMC - PubMed
Dunn 2011 {published data only}
Finer 2010 {published data only}
-
- Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al, SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Early CPAP versus surfactant in extremely preterm infants. New England Journal of Medicine 2010;362(21):1970-9. (Erratum in New England Journal of Medicine 2010; 362(23):2235. [DOI: 10.1056/NEJMoa0911783] [PMID: 20472939] - DOI - PMC - PubMed
Gonçalves‐Ferri 2014 {published data only}
-
- Gonçalves-Ferri WA, Martinez FE, Caldas JP, Marba ST, Fekete S, Rugolo L, et al. Application of continuous positive airway pressure in the delivery room: a multicenter randomized clinical trial. Brazilian Journal of Medical and Biological Research 2014;47(3):259-64. [DOI: 10.1590/1414-431X20133278] [PMID: ] - DOI - PMC - PubMed
Han 1987 {published and unpublished data}
Morley 2008 {published data only}
Sandri 2004 {published data only}
-
- Sandri F, Ancora G, Lanzoni A, Tagliabue P, Colnaghi M, Ventura ML, et al. Prophylactic nasal continuous positive airway pressure in newborns of 28-31 weeks gestation: multicentre randomised controlled clinical trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2004;89(5):F394-8. [DOI: 10.1136/adc.2003.037010] [PMID: ] - DOI - PMC - PubMed
Tapia 2012 {published data only}
-
- Tapia JL, Urzua S, Bancalari A, Meritano J, Torres G, Fabres J, et al, South American Neocosur Network. Randomized trial of early bubble continuous positive airway pressure for very low birth weight infants. Journal of Pediatrics 2012;161:75-80. [DOI: 10.1016/j.jpeds.2011.12.054] [PMID: ] - DOI - PubMed
References to studies excluded from this review
Drew 1982 {published and unpublished data}
Mwatha 2020 {published data only}
-
- Mwatha AB, Mahande M, Olomi R, John B, Philemon R. Treatment outcomes of Pumani bubble-CPAP versus oxygen therapy among preterm babies presenting with respiratory distress at a tertiary hospital in Tanzania—randomised trial. PLoS One 2020;15(6):e0235031. [DOI: 10.1371/journal.pone.0235031] [PMID: ] - DOI - PMC - PubMed
Rojas 2009 {published data only}
-
- Rojas MA, Lozano JM, Ximena-Rojas M, Laughon M, Bose CL, Rondon MA, et al, Colombian Neonatal Research Network. Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: a randomized, controlled trial. Pediatrics 2009;123:137–42. [DOI: 10.1542/peds.2007-3501] [PMID: ] - DOI - PubMed
Thomson 2002 {published data only (unpublished sought but not used)}
-
- Thomson MA, the IFDAS Study Group. Early nasal CPAP + prophylactic surfactant for neonates at risk of RDS. The IFDAS trial. Pediatric Research 2001;50:304A.
-
- Thomson MA. Early nasal continuous positive airways pressure (nCPAP) with prophylactic surfactant for neonates at risk of RDS. The IFDAS multi-centre trial. Pediatric Research 2002;51:379A.
Tooley 2003 {published data only}
Zaharie 2008 {published data only}
-
- Zaharie G, Ion DA, Schmidt N, Popa M, Kudor-Szabadi L, Zaharie T. Prophylactic CPAP versus therapeutic CPAP in preterm newborns of 28-32 gestational weeks. Pneumologia 2008;57(1):34-7. [PMID: ] - PubMed
References to studies awaiting assessment
NCT04209946 {published data only}
-
- NCT04209946. Early caffeine and LISA compared to caffeine and CPAP in preterm infants (CaLI) [A multicenter, randomized trial of preterm infants receiving caffeine and less invasive surfactant administration compared to caffeine and early continuous positive airway pressure (CaLI Trial)]. clinicaltrials.gov/ct2/show/NCT04209946 (first received 24 December 2019).
Additional references
Avery 1987
-
- Avery ME, Tooley WH, Keller JB, Hurd SS, Bryan MH, Cotton RB, et al. Is chronic lung disease in low birth weight infants preventable? A survey of eight centers. Pediatrics 1987;79(1):26-30. [PMID: ] - PubMed
Bancalari 1992
-
- Bancalari E, Sinclair JC. Mechanical ventilation. In: Sinclair JC, Bracken MB, editors(s). Effective Care of the Newborn Infant. Oxford (UK): Oxford University Press, 1992:200-20.
Bayley 1993
-
- Bayley N. Bayley Scales of Infant Development. 2nd edition. San Antonio (TX): The Psychological Corporation, 1993.
Bayley 2006
-
- Bayley N. Bayley Scales of Infant and Toddler Development. San Antonio (TX): Harcourt Assessment, 2006.
Davis 2003
Fanaroff 2013
-
- Fanaroff AA, Fanaroff JM, Klaus MH. Klaus and Fanaroff's Care of the High-Risk Neonate. 6th edition. Philadelphia (PA): Elsevier/Saunders, 2013. [ISBN: 9781416040019]
Gerber 2012
-
- Gerber A, Dargaville PA. Respiratory course and outcome of preterm infants 25-32 weeks gestation managed on nasal continuous positive airway pressure (CPAP) from birth. In: Menzies Research Institute , editors(s). 17th Congress of the Federation of Asian and Oceania Perinatal Societies (FAOPS) and the 16th Annual Congress of the Perinatal Society of Australia and New Zealand (PSANZ); 2012 March; Sydney, NSW, Australia. Hobart (Australia): Wiley Blackwell, 2012:67.
Gittermann 1997
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 27 February 2021. Hamilton (ON): McMaster University (developed by Evidence Prime), 2021. Available at gradepro.org.
Griffiths 1954
-
- Griffiths R. The Abilities of Babies: A Study in Mental Measurement. New York (NY): McGraw-Hill Book Co., Inc, 1954.
Henderson‐Smart 2001
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2020
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Ho 2020a
-
- Ho JJ, Subramaniam P, Sivakaanthan A, Davis PG. Early versus delayed initiation of continuous distending pressure for respiratory distress syndrome in preterm infants. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD002975. [DOI: 10.1002/14651858.CD002975.pub2] - DOI - PMC - PubMed
Ho 2020b
Jacobsen 1993
Jobe 2001
-
- AH Jobe, E Bancalari. Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723-9. [DOI: ] - PubMed
Jonsson 1997
-
- Jonsson B, Katz-Salamon M, Faxelius G, Broberger U, Lagercrantz H. Neonatal care of very-low-birthweight infants in special-care units and neonatal intensive-care units in Stockholm. Early nasal continuous positive airway pressure versus mechanical ventilation: gains and losses. Acta Paediatrica 1997;86(419 Suppl):4-10. [DOI: 10.1111/j.1651-2227.1997.tb18303.x] [PMID: ] - DOI - PubMed
Kattwinkel 1973
-
- Kattwinkel J, Fleming D, Cha CC, Fanaroff AA, Klaus MH. A device for administration of continuous positive airway pressure by the nasal route. Pediatrics 1973;52(1):131-4. [PMID: ] - PubMed
Kattwinkel 2010
-
- Kattwinkel J, Perlman JM, Aziz K, Colby C, Fairchild K, Gallagher J, et al. Neonatal resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics 2010;126(5):e1400-13. - PubMed
Lemyre 2002
-
- Lemyre B, Davis PG, Paoli AG. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for apnea of prematurity. Cochrane Database of Systematic Reviews 2002, Issue 1. Art. No: CD002272. [DOI: 10.1002/14651858.CD002272] [PMID: ] - DOI - PubMed
Lundstrom 1996
-
- Lundstrom KE. Initial treatment of preterm infants - continuous positive airway pressure or ventilation? European Journal of Pediatrics 1996;155(2 Suppl):S25-9. - PubMed
McGoldrick 2020
NIH 1979
-
- National Heart, Lung, and Blood Institute Division of Lung Diseases. Report of Workshop on Bronchopulmonary Dysplasia [National Institutes of Health (NIH). Report of Workshop on Bronchopulmonary Dysplasia]. NIH publication 1979:80-1660. [NIH publication, no. 80-1660]
Olivier 2017
Palisano 1997
Patel 2008
Polin 2002
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.5. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rodriguez 2002
-
- Rodriguez RJ, Martin RJ, Fanaroff AA. Fanaroff and Martin’s Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant. 7th edition. St. Louis (MO): Mosby, 2002.
Schmolzer 2013
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Seger 2009
Soll 1997
Soll 1998
Stevens 2007
-
- Stevens TP, Blennow M, Myers EH, Soll R. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD003063. [DOI: 10.1002/14651858.CD003063.pub3] - DOI - PMC - PubMed
Sweet 2007
-
- Sweet D, Bevilacqua G, Carnielli V, Greisen G, Plavka R, Saugstad OD, et al, Working Group on Prematurity of the World Association of Perinatal Medicine, European Association of Perinatal Medicine. European consensus guidelines on the management of neonatal respiratory distress syndrome. Journal of Perinatal Medicine 2007;35(3):175-86. [DOI: 10.1515/JPM.2007.048] [PMID: ] - DOI - PubMed
Tanswell 1980
References to other published versions of this review
Subramaniam 1998
Subramaniam 2002
Subramaniam 2005
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

