Cutaneous findings following COVID-19 vaccination: review of world literature and own experience
- PMID: 34661927
- PMCID: PMC8656409
- DOI: 10.1111/jdv.17744
Cutaneous findings following COVID-19 vaccination: review of world literature and own experience
Abstract
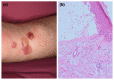
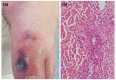
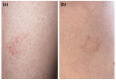
There is growing evidence that not only the novel coronavirus disease (COVID-19) but also the COVID-19 vaccines can cause a variety of skin reactions. In this review article, we provide a brief overview on cutaneous findings that have been observed since the emerging mass COVID-19 vaccination campaigns all over the world. Unspecific injection-site reactions very early occurring after the vaccination are most frequent. Type I hypersensitivity reactions (e.g. urticaria, angio-oedema and anaphylaxis) likely due to allergy to ingredients may rarely occur but can be severe. Type IV hypersensitivity reactions may be observed, including delayed large local skin lesions ("COVID arm"), inflammatory reactions in dermal filler or previous radiation sites or even old BCG scars, and more commonly morbilliform and erythema multiforme-like rashes. Autoimmune-mediated skin findings after COVID-19 vaccination include leucocytoclastic vasculitis, lupus erythematosus and immune thrombocytopenia. Functional angiopathies (chilblain-like lesions, erythromelalgia) may also be observed. Pityriasis rosea-like rashes and reactivation of herpes zoster have also been reported after COVID-19 vaccination. In conclusion, there are numerous cutaneous reaction patterns that may occur following COVID-19 vaccination, whereby many of these skin findings are of immunological/autoimmunological nature. Importantly, molecular mimicry exists between SARS-CoV-2 (e.g. the spike-protein sequences used to design the vaccines) and human components and may thus explain some COVID-19 pathologies as well as adverse skin reactions to COVID-19 vaccinations.
© 2021 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures



Comment in
-
Pityriasis lichenoides chronica after BNT162b2 Pfizer-BioNTech vaccine: A novel cutaneous reaction after SARS-CoV-2 vaccine.J Eur Acad Dermatol Venereol. 2022 Dec;36(12):e979-e981. doi: 10.1111/jdv.18418. Epub 2022 Jul 20. J Eur Acad Dermatol Venereol. 2022. PMID: 35841285 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

