Biomimetic Mechanically Strong One-Dimensional Hydroxyapatite/Poly(d,l-lactide) Composite Inducing Formation of Anisotropic Collagen Matrix
- PMID: 34662097
- PMCID: PMC8613905
- DOI: 10.1021/acsnano.1c03905
Biomimetic Mechanically Strong One-Dimensional Hydroxyapatite/Poly(d,l-lactide) Composite Inducing Formation of Anisotropic Collagen Matrix
Abstract
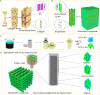
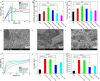
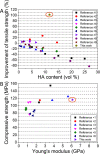
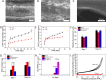
Natural bone is a complex composite, consisting predominantly of collagen and hydroxyapatite (HA), which form a highly organized, hierarchical structure from the nano- to the macroscale. Because of its biphasic, anisotropic, ultrafine structural design, bone tissue possesses excellent mechanical properties. Herein, inspired by the composition and microstructure of natural bone, a biphasic composite consisting of highly aligned strontium/copper-doped one-dimensional hydroxyapatite (Sr/Cu-doped 1D HA) and poly(d,l-lactide) (PDLA) was developed. The presence and alignment of Sr/Cu-doped 1D HA crystals resulted in mechanical reinforcement of the polymer matrix, including compressive and tensile strength and modulus, fracture toughness, swelling resistance, and long-term structural stability. The compressive strength, tensile strength, and Young's modulus of the biomimetic composite were comparable to that of cortical bone. Biologically, the biomimetic composite showed a sustained release of the incorporated Sr and Cu ions, facilitated mineral deposition from simulated body fluid, and supported attachment, proliferation, and alkaline phosphatase activity of human mesenchymal stromal cells (hMSCs). Moreover, the highly aligned Sr/Cu-doped 1D HA crystals in the 3D porous scaffolds induced the alignment of hMSCs and secretion of an anisotropic collagen fiber matrix in 3D. The biomimetic Sr/Cu-doped 1D HA/PDLA composite presented here contributes to the current efforts aiming at the design and development of load-bearing bioactive synthetic bone graft substitutes. Moreover, the biomimetic composite may serve as a 3D platform for studying cell-extracellular matrix interactions in bone tissue.
Keywords: PDLA; anisotropy; biomimetic; bone; composite; hydroxyapatite.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Bohner M.; Miron R. J. A Proposed Mechanism for Material-Induced Heterotopic Ossification. Mater. Today 2019, 22, 132–141. 10.1016/j.mattod.2018.10.036. - DOI
-
- Wu S. L.; Liu X. M.; Yeung K. W. K.; Liu C. S.; Yang X. J. Biomimetic Porous Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng., R 2014, 80, 1–36. 10.1016/j.mser.2014.04.001. - DOI
-
- Woodruff M. A.; Lange C.; Reichert J.; Berner A.; Chen F.; Fratzl P.; Schantz J.-T.; Hutmacher D. W. Bone Tissue Engineering: From Bench to Bedside. Mater. Today 2012, 15 (10), 430–435. 10.1016/S1369-7021(12)70194-3. - DOI
-
- Christy P. N.; Basha S. K.; Kumari V. S.; Bashir A. K. H.; Maaza M.; Kaviyarasu K.; Arasu M. V.; Al-Dhabi N. A.; Ignacimuthu S. Biopolymeric Nanocomposite Scaffolds for Bone Tissue Engineering Applications – A Review. J. Drug Delivery Sci. Technol. 2020, 55, 101452. 10.1016/j.jddst.2019.101452. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

