In Vitro Activity of Ceftibuten/VNRX-5236 against Urinary Tract Infection Isolates of Antimicrobial-Resistant Enterobacterales
- PMID: 34662183
- PMCID: PMC8765315
- DOI: 10.1128/AAC.01304-21
In Vitro Activity of Ceftibuten/VNRX-5236 against Urinary Tract Infection Isolates of Antimicrobial-Resistant Enterobacterales
Abstract
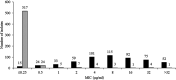
Ceftibuten/VNRX-7145 is a cephalosporin/boronate β-lactamase inhibitor combination under development as an oral treatment for complicated urinary tract infections caused by Enterobacterales producing serine β-lactamases (Ambler class A, C, and D). In vivo, VNRX-7145 (VNRX-5236 etzadroxil) is cleaved to the active inhibitor, VNRX-5236. We assessed the in vitro activity of ceftibuten/VNRX-5236 against 1,066 urinary isolates of Enterobacterales from a 2014-2016 global culture collection. Each isolate tested was preselected to possess a multidrug-resistant (MDR) phenotype that included nonsusceptibility to amoxicillin-clavulanate and resistance to levofloxacin. MICs were determined by CLSI broth microdilution. VNRX-5236 was tested at a fixed concentration of 4 μg/ml. Ceftibuten/VNRX-5236 inhibited 90% of all isolates tested (MIC90) at 2 μg/ml; MIC90s for ESBL- (n = 566), serine carbapenemase- (n = 116), and acquired AmpC-positive (n = 58) isolate subsets were ≤0.25, >32, and 8 μg/ml, respectively. At concentrations of ≤1, ≤2, and ≤4 μg/ml, ceftibuten/VNRX-5236 inhibited 89.1, 91.7, and 93.1% of all isolates tested; 96.5, 97.7, and 98.4% of ESBL-positive isolates; 75.9, 81.9, and 81.9% of serine carbapenemase-positive isolates; and 70.7, 81.0, and 87.9% of acquired AmpC-positive isolates. Ceftibuten/VNRX-5236 at concentrations of ≤1, ≤2, and ≤4 μg/ml inhibited 85-89, 89-91, and 91-92% of isolates that were not susceptible (defined by CLSI and EUCAST breakpoint criteria) to nitrofurantoin, trimethoprim-sulfamethoxazole, and/or fosfomycin, (as part of their MDR phenotype), oral agents commonly prescribed to treat uncomplicated urinary tract infections. The potency of ceftibuten/VNRX-5236 (MIC90, 2 μg/ml) was similar (within one doubling-dilution) to intravenous-only agents ceftazidime-avibactam (MIC90 2 μg/ml) and meropenem-vaborbactam (MIC90 1 μg/ml). Continued investigation of ceftibuten/VNRX-5236 is warranted.
Keywords: Enterobacterales; VNRX-5236; VNRX-7145; ceftibuten; oral therapy; urinary tract infection.
Figures



References
-
- Centers for Disease Control and Prevention. 2019. Antibiotic resistance threats in the United States, 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-re....
-
- World Health Organization. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-E....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

