Effectiveness of the BNT162b2mRNA COVID-19 vaccine in patients with hematological neoplasms in a nationwide mass vaccination setting
- PMID: 34662390
- PMCID: PMC8526123
- DOI: 10.1182/blood.2021013768
Effectiveness of the BNT162b2mRNA COVID-19 vaccine in patients with hematological neoplasms in a nationwide mass vaccination setting
Abstract
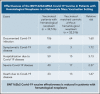
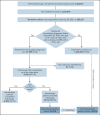
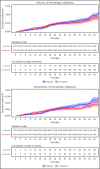
Evidence regarding the effectiveness of COVID-19 vaccine in patients with impaired immunity is limited. Initial observations suggest a lower humoral response in these patients. We evaluated the relative effectiveness of the mRNA BNT162b2 vaccine in patients with hematological neoplasms compared with matched controls. Data on patients with hematological neoplasms after 2 vaccine doses were extracted and matched 1:1 with vaccinated controls. Subpopulation analyses focused on patients receiving therapy for hematological neoplasm, patients without treatment who were only followed, and recipients of specific treatments. The analysis focused on COVID-19 outcomes from days 7 through 43 after the second vaccine dose in these areas: documented COVID-19 infection by polymerase chain reaction; symptomatic infection; hospitalizations; severe COVID-19 disease; and COVID-19-related death. In a population of 4.7 million insured people, 32 516 patients with hematological neoplasms were identified, of whom 5017 were receiving therapy for an active disease. Vaccinated patients with hematological neoplasms, compared with vaccinated matched controls, had an increased risk of documented infections (relative risk [RR] 1.60, 95% CI 1.12-2.37); symptomatic COVID-19 (RR 1.72, 95% CI 1.05-2.85); COVID-19-related hospitalizations (RR 3.13, 95% CI 1.68-7.08); severe COVID-19 (RR 2.27, 95% CI 1.18-5.19); and COVID-19-related death (RR 1.66, 95% CI 0.72-4.47). Limiting the analysis to patients on hematological treatments showed a higher increased risk. This analysis shows that vaccinated patients with hematological neoplasms, in particular patients receiving treatment, suffer from COVID-19 outcomes more than vaccinated individuals with intact immune system. Ways to enhance COVID-19 immunity in this patient population, such as additional doses, should be explored.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures





Comment in
-
Danger ahead: COVID-19 infections after vaccination.Blood. 2022 Mar 10;139(10):1429-1430. doi: 10.1182/blood.2021014505. Blood. 2022. PMID: 35267005 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

