Risk and response adapted de-intensified treatment for HPV-associated oropharyngeal cancer: Optima paradigm expanded experience
- PMID: 34662771
- PMCID: PMC9295443
- DOI: 10.1016/j.oraloncology.2021.105566
Risk and response adapted de-intensified treatment for HPV-associated oropharyngeal cancer: Optima paradigm expanded experience
Abstract
Background: Favorable prognosis for Human papillomavirus-associated (HPV+) oropharyngeal cancer (OPC) led to investigation of response-adaptive de-escalation, yet long-term outcomes are unknown. We present expanded experience and follow-up of risk/response adaptive treatment de-intensification in HPV+ OPC.
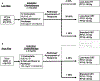
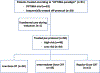
Methods: A phase 2 trial (OPTIMA) and subsequent cohort of sequential off-protocol patients treated from September 2014 to November 2018 at the University of Chicago were reviewed. Eligible patients had T3-T4 or N2-3 (AJCC 7th edition) HPV+ OPC. Patients were stratified by risk: High-risk (HR) (T4, ≥N2c, or >10PYH), all others low-risk (LR). Induction chemotherapy (IC) included 3 cycles of carboplatin and nab-paclitaxel (OPTIMA) or paclitaxel (off-protocol). LR with ≥50% response received low-dose radiotherapy (RT) alone to 50 Gy (RT50). LR with 30-50% response and HR with ≥50% response received intermediate-dose chemoradiotherapy (CRT) to 45 Gy (CRT45). All others received full-dose CRT to 75 Gy (CRT75).
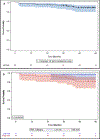
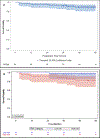
Results: 91 patients consented and 90 patients were treated, of which 31% had >10PYH, 34% had T3/4 disease, and 94% had N2b/N2c/N3 disease. 49% were LR and 51% were HR. Overall response rate to induction was 88%. De-escalated treatment was administered to 83%. Median follow-up was 4.2 years. Five-year OS, PFS, LRC, and DC were 90% (95% CI 81,95), 90% (95% CI 80,95), 96% (95% CI 90,99), and 96% (88,99) respectively. G-tube placement rates in RT50, CRT45, and CRT75 were 3%, 33%, and 80% respectively (p < 0.05).
Conclusion: Risk/response adaptive de-escalated treatment for an inclusive cohort of HPV+ OPC demonstrates excellent survival with reduced toxicity with long-term follow-up.
Keywords: Chemotherapy; Head and neck cancer; Human papillomavirus; Radiation therapy; Treatment de-intensification.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest:
Conflict of Interest Statement:
ZG, EB, JC, DG, AH, JC, SK, CF, NC, ML, EI, and DH report no conflicts of interest.
Figures



References
-
- Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

