Cortisol Deficiency in Lenvatinib Treatment of Thyroid Cancer: An Underestimated Common Adverse Event
- PMID: 34663079
- PMCID: PMC8792496
- DOI: 10.1089/thy.2021.0040
Cortisol Deficiency in Lenvatinib Treatment of Thyroid Cancer: An Underestimated Common Adverse Event
Abstract
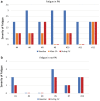
Background: Lenvatinib treatment has shown a significant improvement in progression-free survival in patients with metastatic, progressive, radioiodine-refractory differentiated thyroid cancer, although its use is associated with considerable toxicity. Fatigue is one of the most frequent adverse events (AEs). It has been reported that adrenal insufficiency (AI) may be involved in lenvatinib-related fatigue. In our study, we assessed the pituitary/adrenal axis before and during treatment, and the possible involvement of AI in lenvatinib-related fatigue. This was done to clarify the incidence, development, and time course of AI during lenvatinib treatment. Methods: We studied 13 patients who were selected for lenvatinib therapy. Adrenal function was evaluated by measuring cortisol and adrenocorticotropic hormone (ACTH) levels and through the ACTH (250 μg) stimulation test. Results: During treatment, seven patients (54%) developed AI. High levels of ACTH were observed in accordance with the diagnosis of primary AI (PAI). By evaluating the first ACTH test, before starting lenvatinib treatment, we found that patients with <646.6 nmol/L cortisol peak had an increased risk of developing PAI during lenvatinib treatment. Fatigue was observed in 11 patients (84.6%) during lenvatinib treatment. Cortisone acetate treatment induced an improvement in fatigue in six of seven patients (85.7%) in the PAI group, without the need to change the lenvatinib dosage. Conclusions: PAI may be considered one of the most common AEs associated with lenvatinib. Our data strongly suggest that PAI could be involved in lenvatinib-associated fatigue, particularly in patients with extreme fatigue. In this context, early diagnosis of PAI is essential, especially since glucocorticoid replacement therapy can induce a significant improvement in fatigue, without the need to reduce the dosage of lenvatinib. However, further studies are required to confirm these preliminary findings.
Keywords: fatigue; lenvatinib; primary adrenal insufficiency; thyroid cancer.
Conflict of interest statement
All the authors have nothing to disclose.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
