Ustekinumab trough concentration affects clinical and endoscopic outcomes in patients with refractory Crohn's disease: a Chinese real-world study
- PMID: 34663208
- PMCID: PMC8522105
- DOI: 10.1186/s12876-021-01946-8
Ustekinumab trough concentration affects clinical and endoscopic outcomes in patients with refractory Crohn's disease: a Chinese real-world study
Abstract
Background: Ustekinumab (UST), a newly-used biologic targeting p40 subunit of IL12 and IL23 in China, exerts a confirmed therapeutic effect on the induction and maintenance therapies for refractory Crohn's disease (CD). Therapeutic drug monitoring based on trough and antibody concentration is of core importance when treating patients who lose response to UST. We aimed to analyze the UST exposure-response relationship in CD treatment in the real-world setting.
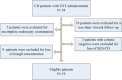
Methods: We retrospectively enrolled patients with CD who received UST between March 1, 2020 and May 31, 2021, at the inflammatory bowel disease (IBD) center of the Sun Yat-Sun Affiliated Sixth Hospital. Baseline characteristic information, biomarker examination, clinical outcomes determined by the Crohn's disease activity index (CDAI), and endoscopic outcomes evaluated using a simple endoscopic score for Crohn's disease (SES-CD) at week 16/20 were collected. The optimal UST cut-off trough concentration was identified using receiver operating characteristic curve (ROC) analysis.
Results: Nineteen eligible patients were included in the study, the mean age was 29.1 ± 9.1 years and the mean disease duration was 5.5 ± 4.7 years. At the initiation of the study, 89.5% of the patients had been exposed to prior biologics, 42.1% had previous CD-related surgeries, and 52.6% had perianal diseases. At week 16/20 after the UST initiation, clinical response, clinical remission, endoscopic response, and endoscopic remission were 89.5%, 84.2%, 42.2%, and 73.7%, respectively. The cut-off optimal trough concentration for UST was 1.12 μg/mL, as determined by the ROC with an area under the curve (AUC) of 0.78, sensitivity of 87.5%, and specificity of 72.7%. Patients with a UST trough concentration > 1.12 μg/mL had a significantly higher rate of endoscopic remission than those without (70.0% vs. 11.1%, P = 0.02).
Conclusions: UST is an effective therapeutic option for refractory CD treatment. A UST trough concentration above 1.12 μg/mL was associated with endoscopic remission at week 16/20 after UST initiation. Trial registration This study was approved and retrospectively registered by the Ethics Committee of Sun Yat-Sen University (2021ZSLYEC-066, March 29, 2021) and the Clinical Trial Registry (NCT04923100, June 10, 2021).
Keywords: Clinical remission; Crohn’s disease; Endoscopic remission; Therapeutic drug monitoring; Trough concentration; Ustekinumab.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Ustekinumab trough levels predicting laboratory and endoscopic remission in patients with Crohn's disease.BMC Gastroenterol. 2022 Apr 21;22(1):195. doi: 10.1186/s12876-022-02271-4. BMC Gastroenterol. 2022. PMID: 35448957 Free PMC article.
-
Association Between Ustekinumab Trough Concentrations and Clinical, Biomarker, and Endoscopic Outcomes in Patients With Crohn's Disease.Clin Gastroenterol Hepatol. 2017 Sep;15(9):1427-1434.e2. doi: 10.1016/j.cgh.2017.03.032. Epub 2017 Mar 29. Clin Gastroenterol Hepatol. 2017. PMID: 28365485
-
Matching-Adjusted Indirect Comparison Between Risankizumab and Ustekinumab for Induction and Maintenance Treatment of Moderately to Severely Active Crohn's Disease.Adv Ther. 2023 Sep;40(9):3896-3911. doi: 10.1007/s12325-023-02546-6. Epub 2023 Jun 27. Adv Ther. 2023. PMID: 37368103 Free PMC article. Clinical Trial.
-
Systematic Review and Meta-analysis: The Association Between Serum Ustekinumab Trough Concentrations and Treatment Response in Inflammatory Bowel Disease.Inflamm Bowel Dis. 2024 Apr 3;30(4):660-670. doi: 10.1093/ibd/izad065. Inflamm Bowel Dis. 2024. PMID: 37071852 Free PMC article.
-
Ustekinumab therapy for Crohn's disease during pregnancy: a case report and review of the literature.J Clin Pharm Ther. 2017 Apr;42(2):234-236. doi: 10.1111/jcpt.12492. Epub 2016 Dec 22. J Clin Pharm Ther. 2017. PMID: 28004853 Review.
Cited by
-
Association Between Serum Ustekinumab Concentrations and Endoscopic Disease Activity in Moderate-to-Severe Crohn's Disease Patients.Crohns Colitis 360. 2024 Nov 29;6(4):otae071. doi: 10.1093/crocol/otae071. eCollection 2024 Oct. Crohns Colitis 360. 2024. PMID: 39668980 Free PMC article.
-
IFX Is Associated with Higher Rates of 2-Year Mucosal Healing in Biologic-Naïve CD Patients Compared to UST.Dig Dis Sci. 2025 Jul 24. doi: 10.1007/s10620-025-09226-1. Online ahead of print. Dig Dis Sci. 2025. PMID: 40707747
-
Ustekinumab in the Treatment of Inflammatory Bowel Diseases: Evolving Paradigms.J Clin Med. 2024 Mar 6;13(5):1519. doi: 10.3390/jcm13051519. J Clin Med. 2024. PMID: 38592377 Free PMC article. Review.
-
Long-term efficacy of therapeutic drug monitoring-guided optimization of ustekinumab maintenance therapy for Crohn's disease patients.Sci Rep. 2025 Jul 15;15(1):25540. doi: 10.1038/s41598-025-09802-5. Sci Rep. 2025. PMID: 40664796 Free PMC article.
-
A Narrative Review of Cytokine Networks: Pathophysiological and Therapeutic Implications for Inflammatory Bowel Disease Pathogenesis.Biomedicines. 2023 Dec 6;11(12):3229. doi: 10.3390/biomedicines11123229. Biomedicines. 2023. PMID: 38137450 Free PMC article. Review.
References
-
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583. doi: 10.1053/j.gastro.2020.12.031. - DOI - PubMed
-
- Casanova MJ, Chaparro M, Minguez M, et al. Effectiveness and safety of the sequential use of a second and third anti-TNF agent in patients with inflammatory bowel disease: results from the eneida registry. Inflamm Bowel Dis. 2020;26(4):606–616. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

