Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biological therapy: 56-week data from the phase 3 SELECT-PsA 1 study
- PMID: 34663636
- PMCID: PMC8524381
- DOI: 10.1136/rmdopen-2021-001838
Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biological therapy: 56-week data from the phase 3 SELECT-PsA 1 study
Erratum in
-
Correction: Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biological therapy: 56-week data from the phase 3 SELECT-PsA 1 study.RMD Open. 2021 Nov;7(3):e001838corr1. doi: 10.1136/rmdopen-2021-001838corr1. RMD Open. 2021. PMID: 34732585 Free PMC article. No abstract available.
Abstract
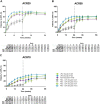
Background: In SELECT-PsA 1, a randomised double-blind phase 3 study, upadacitinib 15 mg and 30 mg were superior to placebo and non-inferior to adalimumab in ≥20% improvement in American College of Rheumatology (ACR) criteria at 12 weeks in patients with psoriatic arthritis (PsA). Here, we report 56-week efficacy and safety in patients from SELECT-PsA 1.
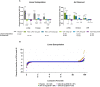
Methods: Patients received upadacitinib 15 mg or 30 mg once daily, adalimumab 40 mg every other week for 56 weeks or placebo through week 24 switched thereafter to upadacitinib 15 mg or 30 mg until week 56. Efficacy endpoints included the proportion of patients achieving ≥20%/50%/70% improvement in ACR criteria (ACR20/50/70), ≥75%/90%/100% improvement in Psoriasis Area and Severity Index (PASI75/90/100), minimal disease activity (MDA) and change from baseline in modified total Sharp/van der Heijde Score. Treatment-emergent adverse events per 100 patient years (PY) were summarised.
Results: Consistent with results through week 24, ACR20/50/70, PASI75/90/100 and MDA responses were maintained with upadacitinib through week 56 and were generally numerically higher than with adalimumab; inhibition of radiographic progression was also maintained. Patients who switched from placebo to upadacitinib exhibited comparable improvements at week 56 as patients originally randomised to upadacitinib. The rates of serious adverse events were 9.1 events/100 PY with upadacitinib 15 mg and 12.3 events/100 PY with upadacitinib 30 mg. Two deaths were reported in each of the upadacitinib groups.
Conclusion: Efficacy across various domains of PsA were maintained with upadacitinib 15 mg and 30 mg through week 56 with no new safety signals observed.
Keywords: adalimumab; arthritis; psoriatic; tumor necrosis factor inhibitors.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: IBM: research grants and honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, Sanofi Regeneron and UCB Pharma. MM: research grants from AbbVie, Amgen and UCB Pharma; consulting fees from Eli Lilly, Janssen, Novartis, Pfizer and UCB Pharma. JFM: consultant and/or investigator for AbbVie, Arena, Avotres, Biogen, Bristol-Myers Squibb, Celgene, Dermavant, Eli Lilly, EMD Sorono, Janssen, Leo Pharma, Merck, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma and UCB Pharma. MK: consulting fees and/or honoraria from AbbVie, AmgenAstellas BioPharma, Asahi-Kasei Pharma, Astellas, Ayumi Pharma, Bristol-Myers Squibb, Chugai, DaiichiSankyo, Eisai, Eli Lilly, Gilead, Janssen, Kyowa Kirin, Novartis, Ono Pharma, Pfizer, Tanabe-Mitsubishi, Teijin Pharma and UCB Pharma. CP-T: research grants and honoraria form AbbVie, AstraZeneca, Eli Lilly, Gilead, Janssen, Pfizer, Roche, R-Pharm, Sanofi Regeneron and UCB Pharma. DH: advisory board/speaker bureau or similar committee for AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Janssen, Novartis, Pfizer, Roche, Sanofi Genzyme and Takeda; funded grant or clinical trials: AbbVie, Adiga Life-Sciences, Amgen, Bristol-Myers Squibb, Can-Fite Biopharma, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Regeneron, Sanofi-Genzyme and UCB Pharma; honoraria or other fees from AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer, Roche, Sanofi Genzyme, Takeda and UCB Pharma; not a part of/has not received payment from a commercial organisation (gifts or ‘in kind’ compensation); does not hold a patent for a product referred to in the CME/CPD program, or marketed by a commercial organisation; does not hold investments in a pharmaceutical organisation, medical devices company or communications firm. FB: research grants from Celgene, Chugai, Janssen, Pfizer and Roche; consultancies/speaker fees from AbbVie, Bristol-Myers Squibb, Boehringer, Celgene, Chugai, Eli Lilly, Genzyme, Janssen, MSD, Novartis, Pfizer, Roche, Sanofi and UCB Pharma. KK, ALP, YD, LC, PZ, RL, JL: AbbVie employees and may own AbbVie stock or options.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
