Molecular insights into mechanisms of GPCR hijacking by Staphylococcus aureus
- PMID: 34663701
- PMCID: PMC8545443
- DOI: 10.1073/pnas.2108856118
Molecular insights into mechanisms of GPCR hijacking by Staphylococcus aureus
Abstract
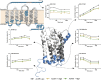
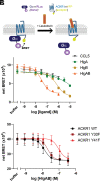
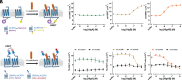
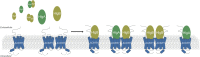
Atypical chemokine receptor 1 (ACKR1) is a G protein-coupled receptor (GPCR) targeted by Staphylococcus aureus bicomponent pore-forming leukotoxins to promote bacterial growth and immune evasion. Here, we have developed an integrative molecular pharmacology and structural biology approach in order to characterize the effect of leukotoxins HlgA and HlgB on ACKR1 structure and function. Interestingly, using cell-based assays and native mass spectrometry, we found that both components HlgA and HlgB compete with endogenous chemokines through a direct binding with the extracellular domain of ACKR1. Unexpectedly, hydrogen/deuterium exchange mass spectrometry analysis revealed that toxin binding allosterically modulates the intracellular G protein-binding domain of the receptor, resulting in dissociation and/or changes in the architecture of ACKR1-Gαi1 protein complexes observed in living cells. Altogether, our study brings important molecular insights into the initial steps of leukotoxins targeting a host GPCR.
Keywords: GPCR; host–pathogen interactions; pharmacology; structural mass spectrometry.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Assis L. M., Nedeljković M., Dessen A., New strategies for targeting and treatment of multi-drug resistant Staphylococcus aureus. Drug Resist. Updat. 31, 1–14 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

