Molecular and genomic characterisation of a panel of human anal cancer cell lines
- PMID: 34663790
- PMCID: PMC8523722
- DOI: 10.1038/s41419-021-04141-5
Molecular and genomic characterisation of a panel of human anal cancer cell lines
Abstract
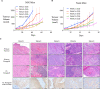
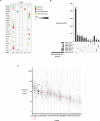
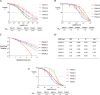
Anal cancer is a rare disease that has doubled in incidence over the last four decades. Current treatment and survival of patients with this disease has not changed substantially over this period of time, due, in part, to a paucity of preclinical models to assess new therapeutic options. To address this hiatus, we set-out to establish, validate and characterise a panel of human anal squamous cell carcinoma (ASCC) cell lines by employing an explant technique using fresh human ASCC tumour tissue. The panel of five human ASCC cell lines were validated to confirm their origin, squamous features and tumourigenicity, followed by molecular and genomic (whole-exome sequencing) characterisation. This panel recapitulates the genetic and molecular characteristics previously described in ASCC including phosphoinositide-3-kinase (PI3K) mutations in three of the human papillomavirus (HPV) positive lines and TP53 mutations in the HPV negative line. The cell lines demonstrate the ability to form tumouroids and retain their tumourigenic potential upon xenotransplantation, with varied inducible expression of major histocompatibility complex class I (MHC class I) and Programmed cell death ligand 1 (PD-L1). We observed differential responses to standard chemotherapy, radiotherapy and a PI3K specific molecular targeted agent in vitro, which correlated with the clinical response of the patient tumours from which they were derived. We anticipate this novel panel of human ASCC cell lines will form a valuable resource for future studies into the biology and therapeutics of this rare disease.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- AIHW. Australian Cancer incidence and Mortality (ACIM) Books: Anal Cancer. Available from http://www.aihw.gov.au/acim-books, 2018.
-
- SEER Cancer Statistics Factsheets: Anal Cancer. National Cancer Institute. Bethesda, MD. 2019. Available from: http://seer.cancer.gov/statfacts/html/anus.html.
-
- Van Der Zee RP, Richel O, De Vries HJC, Prins JM. The increasing incidence of anal cancer: can it be explained by trends in risk groups? Neth J Med. 2013;71:401–12. - PubMed
-
- Meulendijks D, Tomasoa NB, Dewit L, Smits PH, Bakker R, van Velthuysen ML, et al. HPV-negative squamous cell carcinoma of the anal canal is unresponsive to standard treatment and frequently carries disruptive mutations in TP53. Br J Cancer. 2015;112:1358–66. doi: 10.1038/bjc.2015.20. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Postgraduate research scholarship/Department of Health | National Health and Medical Research Council (NHMRC)
- Tour de Cure Scholarship/Royal Australasian College of Surgeons (RACS)
- Foundation for Surgery Research Scholarship/Royal Australasian College of Surgeons (RACS)
- ANZ Journal of Surgery Research Scholarship/Royal Australasian College of Surgeons (RACS)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

