Diurnal changes in the murine small intestine are disrupted by obesogenic Western Diet feeding and microbial dysbiosis
- PMID: 34663882
- PMCID: PMC8523685
- DOI: 10.1038/s41598-021-98986-7
Diurnal changes in the murine small intestine are disrupted by obesogenic Western Diet feeding and microbial dysbiosis
Abstract
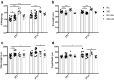
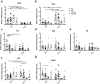
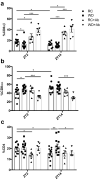
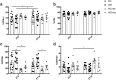
Intestinal functions demonstrate circadian rhythms thought to be entrained, in part, by an organisms' intrinsic feeding and fasting periods as well as by the intestinal microbiome. Circadian disruption as a result of ill-timed nutrient exposure and obesogenic feeding poses an increased risk to disease. As such, the aim of this study was to assess the relationships between dietary timing, composition, and the microbiome with regard to rhythmic small intestinal structure and mucosal immunity. Rodent chow (RC)-mice exhibited time-dependent increases in small intestinal weight, villus height, and crypt depth as well as an increased proportion of CD8αα+ cells and concomitant decrease in CD8αβ+ cells at the onset of the feeding period (p < 0.05-0.001). Western diet (WD)-animals displayed disrupted time-dependent patterns in intestinal structure and lymphocyte populations (p < 0.05-0.01). Antibiotic-induced microbial depletion abrogated the time- and diet-dependent patterns in both RC- and WD-mice (p < 0.05-0.001). However, although germ-free-mice displayed altered rhythms, fecal microbial transfer from RC-mice was generally unsuccessful in restoring structural and immune changes in these animals. This study shows that adaptive changes in the small intestine at the onset of the feeding and fasting periods are disrupted by WD-feeding, and that these changes are dependent, in part, on the intestinal microbiome.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Hoogerwerf WA, Hellmich HL, Cornelissen G, Halberg F, Shahinian VB, Bostwick J, Savidge TC, Cassone VM. Clock gene expression in the murine gastrointestinal tract: Endogenous rhythmicity and effects of a feeding regimen. Gastroenterology. 2007;133:1250–1260. doi: 10.1053/j.gastro.2007.07.009. - DOI - PubMed
-
- Thaiss CA, Zeevi D, Maayan L, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora N, Kuperman Y, Biton I, Gilad S, Harmelin A, Shapiro H, Halpern Z, Segal E, Elinav E. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159:514–529. doi: 10.1016/j.cell.2014.09.048. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

