Comorbidity-associated glutamine deficiency is a predisposition to severe COVID-19
- PMID: 34663907
- PMCID: PMC8522258
- DOI: 10.1038/s41418-021-00892-y
Comorbidity-associated glutamine deficiency is a predisposition to severe COVID-19
Abstract
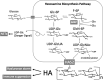
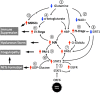
SARS-CoV-2 vaccinations have greatly reduced COVID-19 cases, but we must continue to develop our understanding of the nature of the disease and its effects on human immunity. Previously, we suggested that a dysregulated STAT3 pathway following SARS-Co-2 infection ultimately leads to PAI-1 activation and cascades of pathologies. The major COVID-19-associated metabolic risks (old age, hypertension, cardiovascular diseases, diabetes, and obesity) share high PAI-1 levels and could predispose certain groups to severe COVID-19 complications. In this review article, we describe the common metabolic profile that is shared between all of these high-risk groups and COVID-19. This profile not only involves high levels of PAI-1 and STAT3 as previously described, but also includes low levels of glutamine and NAD+, coupled with overproduction of hyaluronan (HA). SARS-CoV-2 infection exacerbates this metabolic imbalance and predisposes these patients to the severe pathophysiologies of COVID-19, including the involvement of NETs (neutrophil extracellular traps) and HA overproduction in the lung. While hyperinflammation due to proinflammatory cytokine overproduction has been frequently documented, it is recently recognized that the immune response is markedly suppressed in some cases by the expansion and activity of MDSCs (myeloid-derived suppressor cells) and FoxP3+ Tregs (regulatory T cells). The metabolomics profiles of severe COVID-19 patients and patients with advanced cancer are similar, and in high-risk patients, SARS-CoV-2 infection leads to aberrant STAT3 activation, which promotes a cancer-like metabolism. We propose that glutamine deficiency and overproduced HA is the central metabolic characteristic of COVID-19 and its high-risk groups. We suggest the usage of glutamine supplementation and the repurposing of cancer drugs to prevent the development of severe COVID-19 pneumonia.
© 2021. The Author(s), under exclusive licence to ADMC Associazione Differenziamento e Morte Cellulare.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

