A neuropeptide code for itch
- PMID: 34663954
- PMCID: PMC9437842
- DOI: 10.1038/s41583-021-00526-9
A neuropeptide code for itch
Abstract
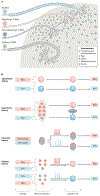
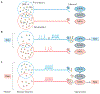
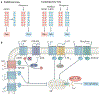
Itch is one of the most primal sensations, being both ubiquitous and important for the well-being of animals. For more than a century, a desire to understand how itch is encoded by the nervous system has prompted the advancement of many theories. Within the past 15 years, our understanding of the molecular and neural mechanisms of itch has undergone a major transformation, and this remarkable progress continues today without any sign of abating. Here I describe accumulating evidence that indicates that itch is distinguished from pain through the actions of itch-specific neuropeptides that relay itch information to the spinal cord. According to this model, classical neurotransmitters transmit, inhibit and modulate itch information in a context-, space- and time-dependent manner but do not encode itch specificity. Gastrin-releasing peptide (GRP) is proposed to be a key itch-specific neuropeptide, with spinal neurons expressing GRP receptor (GRPR) functioning as a key part of a convergent circuit for the conveyance of peripheral itch information to the brain.
© 2021. Springer Nature Limited.
Conflict of interest statement
Competing interests
The author declares no competing interests.
Figures

References
-
- Rothman S Physiology of itching. Physiol. Rev 21, 357–381(1941).
-
- Ikoma A, Steinhoff M, Stander S, Yosipovitch G & Schmelz M The neurobiology of itch. Nat. Rev. Neurosci 7, 535–547 (2006). - PubMed
-
- McMahon SB & Koltzenburg M Itching for an explanation. Trends Neurosci. 15,497–501 (1992). - PubMed
-
- von Frey M Zur Physiologie der Juckempfindung. Arch. Need and. Physiol 7, 142–145 (1922).
-
- Shelley WB & Arthur RP The neurohistology and neurophysiology of the itch sensation in man. AMA Arch. Dermatol 76, 296–323 (1957). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

