Fasting drives the metabolic, molecular and geroprotective effects of a calorie-restricted diet in mice
- PMID: 34663973
- PMCID: PMC8544824
- DOI: 10.1038/s42255-021-00466-9
Fasting drives the metabolic, molecular and geroprotective effects of a calorie-restricted diet in mice
Abstract
Calorie restriction (CR) promotes healthy ageing in diverse species. Recently, it has been shown that fasting for a portion of each day has metabolic benefits and promotes lifespan. These findings complicate the interpretation of rodent CR studies, in which animals typically eat only once per day and rapidly consume their food, which collaterally imposes fasting. Here we show that a prolonged fast is necessary for key metabolic, molecular and geroprotective effects of a CR diet. Using a series of feeding regimens, we dissect the effects of calories and fasting, and proceed to demonstrate that fasting alone recapitulates many of the physiological and molecular effects of CR. Our results shed new light on how both when and how much we eat regulate metabolic health and longevity, and demonstrate that daily prolonged fasting, and not solely reduced caloric intake, is likely responsible for the metabolic and geroprotective benefits of a CR diet.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
COMPETING INTERESTS
D.W.L has received funding from, and is a scientific advisory board member of, Aeovian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases. J.M.D. is a consultant for FORGE Life Sciences and co-founder of Galilei Bio-Sciences. The remaining authors declare no competing interests.
Figures
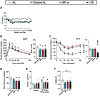




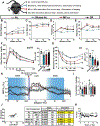



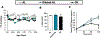
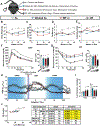



Comment in
-
More evidence of the benefits of fasting.Nat Rev Endocrinol. 2022 Jan;18(1):1. doi: 10.1038/s41574-021-00594-y. Nat Rev Endocrinol. 2022. PMID: 34737439 No abstract available.
References
-
- Das SK et al. Body-composition changes in the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE)-2 study: a 2-y randomized controlled trial of calorie restriction in nonobese humans. Am J Clin Nutr 105, 913–927, doi:10.3945/ajcn.116.137232 (2017). - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- R56 AG056771/AG/NIA NIH HHS/United States
- F31 AG066311/AG/NIA NIH HHS/United States
- I01 BX004031/BX/BLRD VA/United States
- R01 AG056771/AG/NIA NIH HHS/United States
- R01 AG062328/AG/NIA NIH HHS/United States
- S10 OD028739/OD/NIH HHS/United States
- T32 AG000213/AG/NIA NIH HHS/United States
- RF1 AG056771/AG/NIA NIH HHS/United States
- P30 AG050886/AG/NIA NIH HHS/United States
- R01 DK125859/DK/NIDDK NIH HHS/United States
- T32 DK007665/DK/NIDDK NIH HHS/United States
- R37 GM059785/GM/NIGMS NIH HHS/United States
- R21 AG050135/AG/NIA NIH HHS/United States
- R01 DK124696/DK/NIDDK NIH HHS/United States
- R21 AG051974/AG/NIA NIH HHS/United States
- R21 AG061635/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

