Developmentally regulated GTPases: structure, function and roles in disease
- PMID: 34664086
- PMCID: PMC8629797
- DOI: 10.1007/s00018-021-03961-0
Developmentally regulated GTPases: structure, function and roles in disease
Abstract
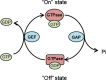
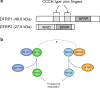
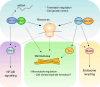
GTPases are a large superfamily of evolutionarily conserved proteins involved in a variety of fundamental cellular processes. The developmentally regulated GTP-binding protein (DRG) subfamily of GTPases consists of two highly conserved paralogs, DRG1 and DRG2, both of which have been implicated in the regulation of cell proliferation, translation and microtubules. Furthermore, DRG1 and 2 proteins both have a conserved binding partner, DRG family regulatory protein 1 and 2 (DFRP1 and DFRP2), respectively, that prevents them from being degraded. Similar to DRGs, the DFRP proteins have also been studied in the context of cell growth control and translation. Despite these proteins having been implicated in several fundamental cellular processes they remain relatively poorly characterized, however. In this review, we provide an overview of the structural biology and biochemistry of DRG GTPases and discuss current understanding of DRGs and DFRPs in normal physiology, as well as their emerging roles in diseases such as cancer.
Keywords: GTPase; Gir2; Rbg1; Rbg2; Ribosome; Tma46; Translation.
© 2021. The Author(s).
Conflict of interest statement
The authors report no competing interests.
Figures



References
-
- Li B, Trueb B. DRG represents a family of two closely related GTP-binding proteins. Biochim Biophys Acta. 2000;1491:196–204. - PubMed
-
- Ishikawa K, Azuma S, Ikawa S, Semba K, Inoue J. Identification of DRG family regulatory proteins (DFRPs): specific regulation of DRG1 and DRG2. Genes Cells. 2005;10:139–150. - PubMed
-
- Leipe DD, Wolf YI, Koonin EV, Aravind L. Classification and evolution of P-loop GTPases and related ATPases1. J Mol Biol. 2002;317:41–72. - PubMed
-
- Wittinghofer A, Vetter IR. Structure-function relationships of the G domain, a canonical switch motif. Annu Rev Biochem. 2011;80:943–971. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

