Antimicrobials from a feline commensal bacterium inhibit skin infection by drug-resistant S. pseudintermedius
- PMID: 34664551
- PMCID: PMC8592530
- DOI: 10.7554/eLife.66793
Antimicrobials from a feline commensal bacterium inhibit skin infection by drug-resistant S. pseudintermedius
Abstract
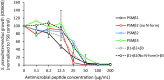
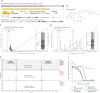
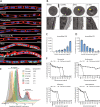
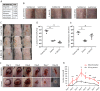
Methicillin-resistant Staphylococcus pseudintermedius (MRSP) is an important emerging zoonotic pathogen that causes severe skin infections. To combat infections from drug-resistant bacteria, the transplantation of commensal antimicrobial bacteria as a therapeutic has shown clinical promise. We screened a collection of diverse staphylococcus species from domestic dogs and cats for antimicrobial activity against MRSP. A unique strain (S. felis C4) was isolated from feline skin that inhibited MRSP and multiple gram-positive pathogens. Whole genome sequencing and mass spectrometry revealed several secreted antimicrobials including a thiopeptide bacteriocin micrococcin P1 and phenol-soluble modulin beta (PSMβ) peptides that exhibited antimicrobial and anti-inflammatory activity. Fluorescence and electron microscopy revealed that S. felis antimicrobials inhibited translation and disrupted bacterial but not eukaryotic cell membranes. Competition experiments in mice showed that S. felis significantly reduced MRSP skin colonization and an antimicrobial extract from S. felis significantly reduced necrotic skin injury from MRSP infection. These findings indicate a feline commensal bacterium that could be utilized in bacteriotherapy against difficult-to-treat animal and human skin infections.
Keywords: B. subtilis; E. coli; Staphylococcus aureus; antimicrobial; human; infection; infectious disease; microbiology; mouse; skin; staphylococcus felis; staphylococcus pseudintermedius.
© 2021, O'Neill et al.
Conflict of interest statement
AO, NK, FL, TN, DM, RM, GK, JC, JN, KP, JP, DG No competing interests declared, KW Dr. Worthing is a co-inventor of technology described in this manuscript that has been disclosed to the University of California San Diego. RG is a co-founder, scientific advisor, consultant and has equity in MatriSys Biosciences and is a consultant, receives income and has equity in Sente
Figures




Similar articles
-
Molecular and phenotypic characterization of methicillin-resistant Staphylococcus pseudintermedius from ocular surfaces of dogs and cats suffering from ophthalmological diseases.Vet Microbiol. 2020 May;244:108687. doi: 10.1016/j.vetmic.2020.108687. Epub 2020 Apr 14. Vet Microbiol. 2020. PMID: 32402352
-
Characterization of Staphylococcal Cassette Chromosome mec Elements from Methicillin-Resistant Staphylococcus pseudintermedius Infections in Australian Animals.mSphere. 2018 Nov 7;3(6):e00491-18. doi: 10.1128/mSphere.00491-18. mSphere. 2018. PMID: 30404937 Free PMC article.
-
Genomic insights into the emergence and spread of methicillin-resistant Staphylococcus pseudintermedius in veterinary clinics.Vet Microbiol. 2021 Jul;258:109119. doi: 10.1016/j.vetmic.2021.109119. Epub 2021 May 15. Vet Microbiol. 2021. PMID: 34023637
-
Antimicrobial resistance in methicillin susceptible and methicillin resistant Staphylococcus pseudintermedius of canine origin: literature review from 1980 to 2013.Vet Microbiol. 2014 Jul 16;171(3-4):337-41. doi: 10.1016/j.vetmic.2014.02.008. Epub 2014 Feb 18. Vet Microbiol. 2014. PMID: 24613081 Review.
-
Review on methicillin-resistant Staphylococcus pseudintermedius.J Antimicrob Chemother. 2011 Dec;66(12):2705-14. doi: 10.1093/jac/dkr367. Epub 2011 Sep 19. J Antimicrob Chemother. 2011. PMID: 21930571 Review.
Cited by
-
Comprehensive Approaches for the Search and Characterization of Staphylococcins.Microorganisms. 2023 May 18;11(5):1329. doi: 10.3390/microorganisms11051329. Microorganisms. 2023. PMID: 37317303 Free PMC article.
-
Angioimmunoblastic T-cell lymphoma: a concise overview encompassing the pathogenetic, pathological, clinical, therapeutical characteristics, and recent advances.Clin Exp Med. 2025 Jun 25;25(1):218. doi: 10.1007/s10238-025-01754-4. Clin Exp Med. 2025. PMID: 40560424 Free PMC article. Review.
-
The porcine skin microbiome exhibits broad fungal antagonism.Fungal Genet Biol. 2024 Aug;173:103898. doi: 10.1016/j.fgb.2024.103898. Epub 2024 May 28. Fungal Genet Biol. 2024. PMID: 38815692 Free PMC article.
-
When the host's away, the pathogen will play: the protective role of the skin microbiome during hibernation.Anim Microbiome. 2023 Dec 21;5(1):66. doi: 10.1186/s42523-023-00285-1. Anim Microbiome. 2023. PMID: 38129884 Free PMC article. Review.
-
Resistance to Critical Important Antibacterials in Staphylococcus pseudintermedius Strains of Veterinary Origin.Antibiotics (Basel). 2022 Dec 5;11(12):1758. doi: 10.3390/antibiotics11121758. Antibiotics (Basel). 2022. PMID: 36551415 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

