A Randomized Study of Lenvatinib 18 mg vs 24 mg in Patients With Radioiodine-Refractory Differentiated Thyroid Cancer
- PMID: 34664662
- PMCID: PMC8852210
- DOI: 10.1210/clinem/dgab731
A Randomized Study of Lenvatinib 18 mg vs 24 mg in Patients With Radioiodine-Refractory Differentiated Thyroid Cancer
Abstract
Background: Lenvatinib is a multikinase inhibitor approved to treat radioiodine-refractory differentiated thyroid cancer (RR-DTC) at a starting dose of 24 mg/day. This study explored, in a double-blinded fashion, whether a starting dose of 18 mg/day would provide comparable efficacy with reduced toxicity.
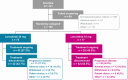
Methods: Patients with RR-DTC were randomized to lenvatinib 24 mg/day or 18 mg/day. The primary efficacy endpoint was objective response rate as of week 24 (ORRwk24); the odds ratio noninferiority margin was 0.4. The primary safety endpoint was frequency of grade ≥3 treatment-emergent adverse events (TEAEs) as of week 24. Tumors were assessed using RECIST v1.1. TEAEs were monitored and recorded.
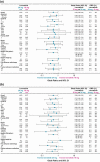
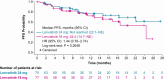
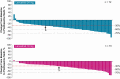
Results: The ORRwk24 was 57.3% (95% CI 46.1, 68.5) in the lenvatinib 24-mg arm and 40.3% (95% CI 29.3, 51.2) in the lenvatinib 18-mg arm, with an odds ratio (18/24 mg) of 0.50 (95% CI 0.26, 0.96). As of week 24, the rates of TEAEs grade ≥3 were 61.3% in the lenvatinib 24-mg arm and 57.1% in the lenvatinib 18-mg arm, a difference of -4.2% (95% CI -19.8, 11.4).
Conclusion: A starting dose of lenvatinib 18 mg/day did not demonstrate noninferiority compared to a starting dose of 24 mg/day as assessed by ORRwk24 in patients with RR-DTC. The results represent a clinically meaningful difference in ORRwk24. The safety profile was comparable, with no clinically relevant difference between arms. These results support the continued use of the approved starting dose of lenvatinib 24 mg/day in patients with RR-DTC and adjusting the dose as necessary.
Trial registration: ClinicalTrials.gov NCT02702388.
Keywords: RR-DTC; lenvatinib; starting dose; tyrosine kinase inhibitor.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society.
Figures
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. - PubMed
-
- Cabanillas ME, Habra MA. Lenvatinib: role in thyroid cancer and other solid tumors. Cancer Treat Rev. 2016;42:47-55. - PubMed
-
- Eustatia-Rutten CF, Corssmit EP, Biermasz NR, Pereira AM, Romijn JA, Smit JW. Survival and death causes in differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2006;91(1):313-319. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

