The Birth of Genomic Enzymology: Discovery of the Mechanistically Diverse Enolase Superfamily
- PMID: 34664940
- PMCID: PMC10251251
- DOI: 10.1021/acs.biochem.1c00494
The Birth of Genomic Enzymology: Discovery of the Mechanistically Diverse Enolase Superfamily
Abstract
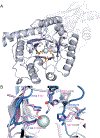
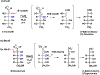
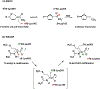
Enzymes are categorized into superfamilies by sequence, structural, and mechanistic similarities. The evolutionary implications can be profound. Until the mid-1990s, the approach was fragmented largely due to limited sequence and structural data. However, in 1996, Babbitt et al. published a paper in Biochemistry that demonstrated the potential power of mechanistically diverse superfamilies to identify common ancestry, predict function, and, in some cases, predict specificity. This Perspective describes the findings of the original work and reviews the current understanding of structure and mechanism in the founding family members. The outcomes of the genomic enzymology approach have reached far beyond the functional assignment of members of the enolase superfamily, inspiring the study of superfamilies and the adoption of sequence similarity networks and genome context and yielding fundamental insights into enzyme evolution.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
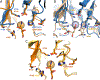


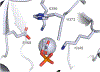




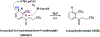

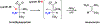

References
-
- Babbitt PC, Hasson MS, Wedekind JE, Palmer DR, Barrett WC, Reed GH, Rayment I, Ringe D, Kenyon GL, and Gerlt JA (1996) The enolase superfamily: a general strategy for enzyme-catalyzed abstraction of the alpha-protons of carboxylic acids, Biochemistry 35, 16489–16501. - PubMed
-
- Finer-Moore JS, Santi DV, and Stroud RM (2003) Lessons and conclusions from dissecting the mechanism of a bisubstrate enzyme: thymidylate synthase mutagenesis, function, and structure, Biochemistry 42, 248–256. - PubMed
-
- Moniot S, Bruno S, Vonrhein C, Didierjean C, Boschi-Muller S, Vas M, Bricogne G, Branlant G, Mozzarelli A, and Corbier C (2008) Trapping of the thioacylglyceraldehyde-3-phosphate dehydrogenase intermediate from Bacillus stearothermophilus. Direct evidence for a flip-flop mechanism, J Biol Chem 283, 21693–21702. - PubMed
-
- Leung D, Abbenante G, and Fairlie DP (2000) Protease inhibitors: current status and future prospects, J Med Chem 43, 305–341. - PubMed
-
- Landro JA, Gerlt JA, Kozarich JW, Koo CW, Shah VJ, Kenyon GL, Neidhart DJ, Fujita S, and Petsko GA (1994) The role of lysine 166 in the mechanism of mandelate racemase from Pseudomonas putida: mechanistic and crystallographic evidence for stereospecific alkylation by (R)-alpha-phenylglycidate, Biochemistry 33, 635–643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

