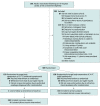
Effect of Moderate vs Mild Therapeutic Hypothermia on Mortality and Neurologic Outcomes in Comatose Survivors of Out-of-Hospital Cardiac Arrest: The CAPITAL CHILL Randomized Clinical Trial
- PMID: 34665203
- PMCID: PMC8527358
- DOI: 10.1001/jama.2021.15703
Effect of Moderate vs Mild Therapeutic Hypothermia on Mortality and Neurologic Outcomes in Comatose Survivors of Out-of-Hospital Cardiac Arrest: The CAPITAL CHILL Randomized Clinical Trial
Abstract
Importance: Comatose survivors of out-of-hospital cardiac arrest experience high rates of death and severe neurologic injury. Current guidelines recommend targeted temperature management at 32 °C to 36 °C for 24 hours. However, small studies suggest a potential benefit of targeting lower body temperatures.
Objective: To determine whether moderate hypothermia (31 °C), compared with mild hypothermia (34 °C), improves clinical outcomes in comatose survivors of out-of-hospital cardiac arrest.
Design, setting, and participants: Single-center, double-blind, randomized, clinical superiority trial carried out in a tertiary cardiac care center in eastern Ontario, Canada. A total of 389 patients with out-of-hospital cardiac arrest were enrolled between August 4, 2013, and March 20, 2020, with final follow-up on October 15, 2020.
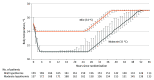
Interventions: Patients were randomly assigned to temperature management with a target body temperature of 31 °C (n = 193) or 34 °C (n = 196) for a period of 24 hours.
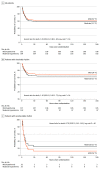
Main outcomes and measures: The primary outcome was all-cause mortality or poor neurologic outcome at 180 days. Neurologic outcome was assessed using the Disability Rating Scale, with poor neurologic outcome defined as a score greater than 5 (range, 0-29, with 29 being the worst outcome [vegetative state]). There were 19 secondary outcomes, including mortality at 180 days and length of stay in the intensive care unit.
Results: Among 367 patients included in the primary analysis (mean age, 61 years; 69 women [19%]), 366 (99.7%) completed the trial. The primary outcome occurred in 89 of 184 patients (48.4%) in the 31 °C group and in 83 of 183 patients (45.4%) in the 34 °C group (risk difference, 3.0% [95% CI, 7.2%-13.2%]; relative risk, 1.07 [95% CI, 0.86-1.33]; P = .56). Of the 19 secondary outcomes, 18 were not statistically significant. Mortality at 180 days was 43.5% and 41.0% in patients treated with a target temperature of 31 °C and 34 °C, respectively (P = .63). The median length of stay in the intensive care unit was longer in the 31 °C group (10 vs 7 days; P = .004). Among adverse events in the 31 °C group vs the 34 °C group, deep vein thrombosis occurred in 11.4% vs 10.9% and thrombus in the inferior vena cava occurred in 3.8% and 7.7%, respectively.
Conclusions and relevance: In comatose survivors of out-of-hospital cardiac arrest, a target temperature of 31 °C did not significantly reduce the rate of death or poor neurologic outcome at 180 days compared with a target temperature of 34 °C. However, the study may have been underpowered to detect a clinically important difference.
Trial registration: ClinicalTrials.gov Identifier: NCT02011568.
Conflict of interest statement
Figures