Identifying key regulators of the intestinal stem cell niche
- PMID: 34665221
- PMCID: PMC8589435
- DOI: 10.1042/BST20210223
Identifying key regulators of the intestinal stem cell niche
Abstract
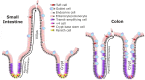
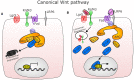
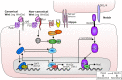
The intestinal tract is lined by a single layer of epithelium that is one of the fastest regenerating tissues in the body and which therefore requires a very active and exquisitely controlled stem cell population. Rapid renewal of the epithelium is necessary to provide a continuous physical barrier from the intestinal luminal microenvironment that contains abundant microorganisms, whilst also ensuring an efficient surface for the absorption of dietary components. Specialised epithelial cell populations are important for the maintenance of intestinal homeostasis and are derived from adult intestinal stem cells (ISCs). Actively cycling ISCs divide by a neutral drift mechanism yielding either ISCs or transit-amplifying epithelial cells, the latter of which differentiate to become either absorptive lineages or to produce secretory factors that contribute further to intestinal barrier maintenance or signal to other cellular compartments. The mechanisms controlling ISC abundance, longevity and activity are regulated by several different cell populations and signalling pathways in the intestinal lamina propria which together form the ISC niche. However, the complexity of the ISC niche and communication mechanisms between its different components are only now starting to be unravelled with the assistance of intestinal organoid/enteroid/colonoid and single-cell imaging and sequencing technologies. This review explores the interaction between well-established and emerging ISC niche components, their impact on the intestinal epithelium in health and in the context of intestinal injury and highlights future directions and implications for this rapidly developing field.
Keywords: epithelial dynamics; gastrointestinal physiology; intestinal epithelial cells; intestinal homeostasis; intestinal stem cell niche; intestinal stem cells.
© 2021 The Author(s).
Conflict of interest statement
The author declares that there are no competing interests associated with this manuscript.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

