Hyperammonemia in Inherited Metabolic Diseases
- PMID: 34665389
- PMCID: PMC11421644
- DOI: 10.1007/s10571-021-01156-6
Hyperammonemia in Inherited Metabolic Diseases
Abstract
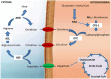
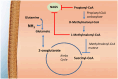
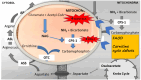
Ammonia is a neurotoxic compound which is detoxified through liver enzymes from urea cycle. Several inherited or acquired conditions can elevate ammonia concentrations in blood, causing severe damage to the central nervous system due to the toxic effects exerted by ammonia on the astrocytes. Therefore, hyperammonemic patients present potentially life-threatening neuropsychiatric symptoms, whose severity is related with the hyperammonemia magnitude and duration, as well as the brain maturation stage. Inherited metabolic diseases caused by enzymatic defects that compromise directly or indirectly the urea cycle activity are the main cause of hyperammonemia in the neonatal period. These diseases are mainly represented by the congenital defects of urea cycle, classical organic acidurias, and the defects of mitochondrial fatty acids oxidation, with hyperammonemia being more severe and frequent in the first two groups mentioned. An effective and rapid treatment of hyperammonemia is crucial to prevent irreversible neurological damage and it depends on the understanding of the pathophysiology of the diseases, as well as of the available therapeutic approaches. In this review, the mechanisms underlying the hyperammonemia and neurological dysfunction in urea cycle disorders, organic acidurias, and fatty acids oxidation defects, as well as the therapeutic strategies for the ammonia control will be discussed.
Keywords: Ammonia; Defects of fatty acids oxidation; Hyperammonemia; Organic acidurias; Urea cycle disorders.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare there are no conflicts of interest.
Figures
References
-
- Acharya SK, Bhatia V, Sreenivas V, Khanal S, Panda SK (2009) Efficacy of l-ornithine l-aspartate in acute liver failure: a double-blind, randomized, placebo-controlled study. Gastroenterology 136(7):2159–2168. 10.1053/j.gastro.2009.02.050 - PubMed
-
- Albrecht J, Norenberg MD (2006) Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology 44(4):788–794. 10.1002/hep.21357 - PubMed
-
- Alger BE, Nicoll RA (1983) Ammonia does not selectively block IPSPs in rat hippocampal pyramidal cells. J Neurophysiol 49(6):1381–1391. 10.1152/jn.1983.49.6.1381 - PubMed
-
- Anderson DR, Viau K, Botto LD, Pasquali M, Longo N (2020) Clinical and biochemical outcomes of patients with medium-chain acyl-CoA dehydrogenase deficiency. Mol Genet Metab 129:13–19. 10.1016/j.ymgme.2019.11.006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

