The natural history of 21-hydroxylase autoantibodies in autoimmune Addison's disease
- PMID: 34665570
- PMCID: PMC8052519
- DOI: 10.1530/EJE-20-1268
The natural history of 21-hydroxylase autoantibodies in autoimmune Addison's disease
Abstract
Background: The most common cause of primary adrenal failure (Addison's disease) in the Western world is autoimmunity characterized by autoantibodies against the steroidogenic enzyme 21-hydroxylase (CYP21A2, 21OH). Detection of 21OH-autoantibodies is currently used for aetiological diagnosis, but how levels of 21OH-autoantibodies vary over time is not known.
Setting: Samples from the national Norwegian Addison's Registry and Biobank established in 1996 (n = 711). Multi-parameter modelling of the course of 21OH-autoantibody indices over time.
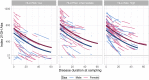
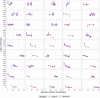
Results: 21OH-autoantibody positivity is remarkably stable, and >90% of the patients are still positive 30 years after diagnosis. Even though the antibody levels decline with disease duration, it is only rarely that this downturn reaches negativity. 21OH-autoantibody indices are affected by age at diagnosis, sex, type of Addison's disease (isolated vs autoimmune polyendocrine syndrome type I or II) and HLA genotype.
Conclusion: 21OH-autoantibodies are reliable and robust markers for autoimmune Addison's disease, linked to HLA risk genotype. However, a negative test in patients with long disease duration does not exclude autoimmune aetiology.
© 2021 The Authors.
Figures


References
-
- Husebye ES, Allolio B, Arlt W, Badenhoop K, Bensing S, Betterle C, Falorni A, Gan EH, Hulting AL, Kasperlik-Zaluska Aet al. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. Journal of Internal Medicine 2014. 275 104–115. (10.1111/joim.12162) - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

