Light-weight electrophysiology hardware and software platform for cloud-based neural recording experiments
- PMID: 34666315
- PMCID: PMC8667733
- DOI: 10.1088/1741-2552/ac310a
Light-weight electrophysiology hardware and software platform for cloud-based neural recording experiments
Abstract
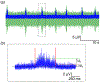
Objective.Neural activity represents a functional readout of neurons that is increasingly important to monitor in a wide range of experiments. Extracellular recordings have emerged as a powerful technique for measuring neural activity because these methods do not lead to the destruction or degradation of the cells being measured. Current approaches to electrophysiology have a low throughput of experiments due to manual supervision and expensive equipment. This bottleneck limits broader inferences that can be achieved with numerous long-term recorded samples.Approach.We developed Piphys, an inexpensive open source neurophysiological recording platform that consists of both hardware and software. It is easily accessed and controlled via a standard web interface through Internet of Things (IoT) protocols.Main results.We used a Raspberry Pi as the primary processing device along with an Intan bioamplifier. We designed a hardware expansion circuit board and software to enable voltage sampling and user interaction. This standalone system was validated with primary human neurons, showing reliability in collecting neural activity in near real-time.Significance.The hardware modules and cloud software allow for remote control of neural recording experiments as well as horizontal scalability, enabling long-term observations of development, organization, and neural activity at scale.
Keywords: IoT; data acquisition; electrophysiology; in vitro; neural recording; open source; scalable.
Creative Commons Attribution license.
Conflict of interest statement
Competing interests
The authors declare no conflict of interest.
Figures







References
-
- Hansel D, Mato G, and Meunier C. Synchrony in Excitatory Neural Networks. Neural Computation, 7(2):307–337, March 1995. Publisher: MIT Press. - PubMed
-
- Trujillo Cleber A., Gao Richard, Negraes Priscilla D., Gu Jing, Buchanan Justin, Preissl Sebastian, Wang Allen, Wu Wei, Haddad Gabriel G., Chaim Isaac A., Domissy Alain, Vandenberghe Matthieu, Devor Anna, Yeo Gene W., Voytek Bradley, and Muotri Alysson R.. Complex Oscillatory Waves Emerging from Cortical Organoids Model Early Human Brain Network Development. Cell Stem Cell, 25(4):558–569.e7, October 2019. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
