Severe cytokine release syndrome is associated with hematologic toxicity following CD19 CAR T-cell therapy
- PMID: 34666344
- PMCID: PMC9006285
- DOI: 10.1182/bloodadvances.2020004142
Severe cytokine release syndrome is associated with hematologic toxicity following CD19 CAR T-cell therapy
Abstract
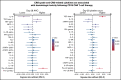
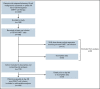
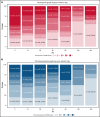
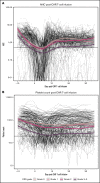
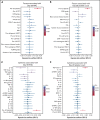
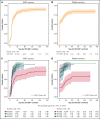
CD19-targeted chimeric antigen receptor (CAR) T-cell therapy has demonstrated remarkable efficacy in patients with relapsed/refractory B-cell malignancies; however, it is associated with toxicities including cytokine release syndrome (CRS), neurotoxicity, and impaired hematopoietic recovery. The latter is associated with high-grade cytopenias requiring extended growth factor or transfusional support, potentially leading to additional complications such as infection or hemorrhage. To date, the factors independently associated with hematologic toxicity have not been well characterized. To address this deficit, we retrospectively analyzed 173 patients who received defined-composition CD19 CAR T-cell therapy in a phase 1/2 clinical trial (https://clinicaltrials.gov; NCT01865617), with primary end points of absolute neutrophil count and platelet count at day-28 after CAR T-cell infusion. We observed cumulative incidences of neutrophil and platelet recovery of 81% and 75%, respectively, at 28 days after infusion. Hematologic toxicity was noted in a significant subset of patients, with persistent neutropenia in 9% and thrombocytopenia in 14% at last follow-up. Using debiased least absolute shrinkage selector and operator regression analysis for high-dimensional modeling and considering patient-, disease-, and treatment-related variables, we identified increased CRS severity as an independent predictor for decreased platelet count and lower prelymphodepletion platelet count as an independent predictor of both decreased neutrophil and platelet counts after CD19 CAR T-cell infusion. Furthermore, multivariable models including CRS-related cytokines identified associations between higher peak serum concentrations of interleukin-6 and lower day-28 cell counts; in contrast, higher serum concentrations of transforming growth factor-β1 were associated with higher counts. Our findings suggest that patient selection and improved CRS management may improve hematopoietic recovery after CD19 CAR T-cell therapy.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

