Pretherapy metabolic tumor volume is associated with response to CD30 CAR T cells in Hodgkin lymphoma
- PMID: 34666347
- PMCID: PMC8864661
- DOI: 10.1182/bloodadvances.2021005385
Pretherapy metabolic tumor volume is associated with response to CD30 CAR T cells in Hodgkin lymphoma
Abstract
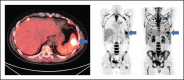
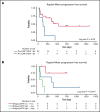
Our group has recently demonstrated that chimeric antigen receptor T-cell therapy targeting the CD30 antigen (CD30.CAR-T) is highly effective in patients with relapsed and refractory (r/r) classical Hodgkin lymphoma (cHL). Despite high rates of clinical response, relapses and progression were observed in a subset of patients. The objective of this study was to characterize clinical and correlative factors associated with progression-free survival (PFS) after CD30.CAR-T cell therapy. We evaluated correlatives in 27 patients with r/r cHL treated with lymphodepletion and CD30.CAR-T cells. With a median follow-up of 9.5 months, 17 patients (63%) progressed, with a median PFS of 352 days (95% confidence interval: 116-not reached), and 2 patients died (7%) with a median overall survival of not reached. High metabolic tumor volume (MTV, >60 mL) immediately before lymphodepletion and CD30.CAR-T cell infusion was associated with inferior PFS (log rank, P = .02), which persisted after adjusting for lymphodepletion and CAR-T dose (log rank, P = .01 and P = .006, respectively). In contrast, receiving bridging therapy, response to bridging therapy, CD30.CAR-T expansion/persistence, and percentage of CD3+PD-1+ lymphocytes over the first 6 weeks of therapy were not associated with differences in PFS. In summary, this study reports an association between high baseline MTV immediately before lymphodepletion and CD30.CAR-T cell infusion and worse PFS in patients with r/r cHL. This trial was registered at www.clinicaltrials.gov as #NCT02690545.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures


References
-
- Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators . Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. - PubMed
-
- Munshi NC, Anderson LD Jr, Shah N, et al. . Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705-716. - PubMed

